Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Biomedical
As a new type of one-dimensional semiconductor nanometer material, silicon nanowires (SiNWs) possess good application prospects in the field of biomedical sensing. SiNWs have excellent electronic properties for improving the detection sensitivity of biosensors. The combination of SiNWs and field effect transistors (FETs) formed one special biosensor with high sensitivity and target selectivity in real-time and label-free. Recently, SiNW-FETs have received more attention in fields of biomedical detection.
- silicon nanowires (SiNWs)
- field effect transistors (FETs)
- biosensor
1. DNA Determination
Deoxyribonucleic acid (DNA), one of the most important genetic materials, functions not only as carriers for transferring genetic information, but also as ideal biomarkers for clinical diagnosis. As a good candidate for monitoring DNA/RNA hybridizations with ultra-high sensitivity (femtomolar level), SiNW-FET has higher integration and label-free detection abilities than surface plasmon resonance (SPR) [41] and quartz crystal microbalance [42].
Many disease biomarkers are DNA molecules like hepatitis B virus, avian influenza virus, cancer, and others. The detection of specific DNA is extremely important from the perspective of medical diagnoses. For example, the early diagnosis of cancer is crucial to a patient’s life. Li et al. [43] achieved the detection of tumor marker circulating tumor DNA (ctDNA) with a sensitivity of 10 aM using an inverted triangle SiNW array FET (Figure 1a). The cross-sectional dimensions of SiNWs were approximately 90 nm with a deviation less than ±20 nm. Compared with a single SiNW, 120 SiNWs with a high consistency of dimensions enabled the superposition of signals, resulting in the much stronger response signal and a higher signal-to-noise ratio.
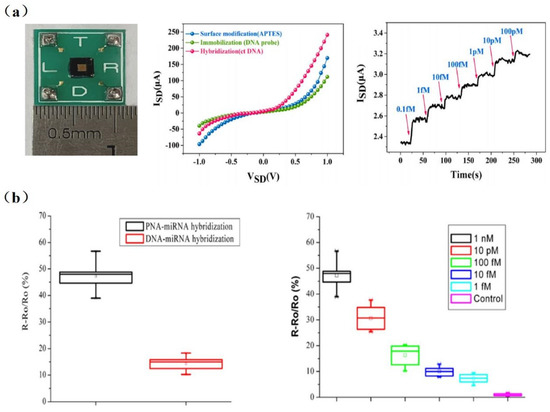
Figure 1. (a) Left to right: Photograph of packaged SiNW-array FET devices; I−V characteristics of the same SiNW-array FET sensor at different stage; the real-time response of SiNW-array FET biosensor for different concentrations of ctDNA (from 0.1 fM to 100 pM). Red arrows indicate the points of injections [43]. (b) Left to right: The response comparison of DNA-functionalized and PNA-functionalized SiNW biosensors to detect complementary miRNAs; the response of PNA-functionalized SiNW biosensors to different concentrations of complementary miRNAs. Its detection can be as low as 1 fM [32].
Gao et al. [25] detected the DNA of two pathogenic strains of avian influenza (H1N1 and H5N1) by immobilizing two DNA probes on the surface of SiNW, respectively. Their experimental results showed that the current was related to the logarithm of the DNA concentration. Lin et al. [44] used a Poly-SiNW FET to achieve the specific and ultra-sensitive (fM level) detection of pathogenic avian influenza virus (H5 and H7) DNA.
Due to the large amount of negative charges in the phosphate backbone of DNA/RNA, the charge carrier density of SiNW-FET biosensors varies significantly during DNA or RNA hybridization [19,45]. Although peptide nucleic acid (PNA) has no phosphate groups in its backbone, it was proven to be capable of determining the distance between target–receptor binding sites and the SiNW surface. As shown in Figure 1b, the binding of PNA/DNA or PNA/RNA strands is stronger than DNA/DNA or DNA/RNA. Hahm et al. [19] demonstrated that PNA-DNA duplex formation has successfully contributed to the detection of DNA in real time. They observed a considerable increase in conductance in the p-type PNA-modified SiNW-FET, with the detection limit down to 10 fM. PNA-based SiNW-FETs are a promising detection platform for various physiological applications [34]. Zhang et al. [32] achieved a detection limit down to 1 fM for miRNA sensing in PNA/SiNW-FET (Figure 1b). It is able to identify miRNAs from the whole RNA extract of HeLa cells, which will contribute to the early diagnosis of tumors. DNA extraction is not necessary for testing ctDNA in blood, while it is required before some viral or cellular nucleic acid assays.
Wenga, G. et al. [46] proposed a novel stepped polycrystalline silicon nanowire field effect transistor for the ultrasensitive detection of DNA hybridization. DNA probes were immobilized on the surface of polycrystalline silicon nanowires that were synthesized by sidewall spacer technology to detect DNA targets. The experimental results showed that the detection limit of complementary DNA targets could be 1 fM.
Due to the unique advantages of DNA analogues as probes for detecting target DNA, more and more scholars have begun to pay attention to DNA analogs. Hu, W.P., and colleagues [47] found that the configured neutralizing of DNA analogues as probes for target DNA can increase the Debye length, which is beneficial for improving the sensitivity and signal-to-noise ratio of the biosensor. They synthesized two partially neutralized chimeric DNA products and a fully neutralized DNA sequence using phosphomethylated nucleotides. The design of the neutralizing DNA probe reduced the ion solubility required for hybridization with the target DNA and increased the Debye length. In addition, the uncharged DNA probes avoided charge repulsion. A BTP buffer was also used to reduce the Debye shielding effect. Therefore, the detection limit of the SiNW-FET was as low as 0.1 fM.
In order to increase the sensitivity and repeatability of the SiNW-FET sensor, Mikael et al. [48] demonstrated repeatable and efficient DNA hybridization detection by coating the SiNW’s surface with a thin layer of HfO2. In a dry environment, the SiNWs were passivated by HfO2, and the DNA probes were transplanted onto the surface. Because HfO2 had a higher density of OH groups and a high dielectric constant, the density of DNA hybridization detected by this sensor was 1010 cm−2.
Now, SiNW-FET biosensors have been regarded as promising tools in DNA detection due to their label-free, ultra-highly sensitive, and rapid detection capabilities. As discussed above, SiNW-FET has been applied to detect DNA hybridization, infectious viruses, and miRNAs. However, further optimization of the device is still needed, such as reducing the width and length of the sensor or reducing unnecessary procedures to improve the sensitivity and accuracy of nanoscale devices.
Xie et al. [49] constructed an independent and integrated microfluidic nanodetection system that incorporates SiNW-FET biosensors for biodetection and analysis. All analytical processes, such as liquid sample delivery, thermostatic control, signal amplification, data acquisition, and result display, are performed automatically. This portable nanosensor detection system is mainly composed of five parts: the liquid circuit module, the light modulation module, the constant temperature control module, the signal acquisition and amplification module, and the status and result display module. The system can effectively solve the problem that SiNW-FET biosensors usually require discrete detectors and have not yet been implemented in the integrated and mobile fields. Because the surfaces of SiNW-FET biosensors are inevitably exposed to the external environment, they are susceptible to interference. They are extremely sensitive to external environmental factors such as temperature, light, and pH, which can cause detection errors. Therefore, SiNW-FET is expected to detect biomarkers in field applications in the near future.
2. Protein Detection
Protein is an important component of human cells and tissues, which plays a significant role in human life activities. Proteins realize the expression of genes under the control of DNA, which make organisms exhibit a variety of genetic characteristics. Many biomarkers used in disease diagnosis are proteins [17,50]. Therefore, achieving effective and sensitive detection of proteins as biomarkers is of great significance for the disease diagnosis.
The Enzyme-Linked Immunosorbent Assay (ELISA) [51], laser, and fluorescence technology [52] have all been used for the detection of proteins. Protein detection and separation technology have been constantly evolving due to improvements in microelectronic technology. Nowadays, more and more researchers are focusing on SiNW-FETs, which are suitable for protein detection.
The real-time detection of biotin using an SiNW-FET biosensor was first achieved in 2001 by Cui et al. [5]. And, the detection limit reached 10 pmol/L, which was far lower than others test method. However, it was not possible to continuously detect samples with different concentrations due to the irreversible combination of biotin and streptomycin. Kim et al. [53] proposed the combination of a conventional immunoassay (Sandwich ELISA) with high-precision FET devices and accomplished the multichannel detection of three different cancer markers (carcinoembryonic antigen (CEA), prostate specific antigen (PSA), and alpha fetoprotein (AFP)) in serum without pretreatment successfully. As illustrated in Figure 2, four different antibodies were self-assembled on the chip surface (three tumor markers PSA, CEA, AFP, and monoclonal antibodies (IgG)). Then, serum antigens at concentrations of 1 and 10 ng/mL were injected into groups with tumor markers fixed, and changes before and after the injection were observed. The detection limit was as low as ng/mL level.
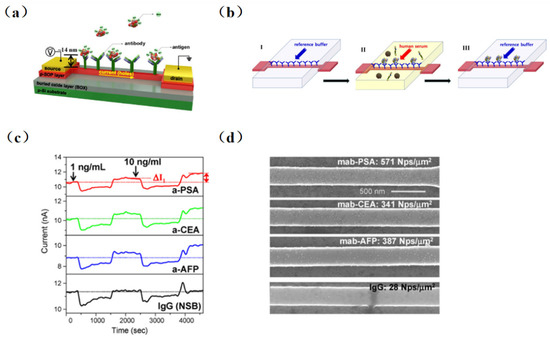
Figure 2. Biosensor schematic diagram and sketch of new sensing method in physiological solution. (a) Immobilization of antibodies on the sensing channel between the source and drain electrodes to detect negatively charged antigens. (b) Schematic diagram of the novel immunoassay device. In step I, a reference buffer solution (10 μM phosphate, 20 μM NaCl, pH 7.8) is injected to fix the antibody on the surface of the sensor. Step II is for Ag–Ab interaction of target antigen (cancer marker) in the serum containing the immobilized antibody. Step III: the target antigen is bound to the antibody fixed on the FET sensor by buffer solution washing out the human serum, and the electrical measurement can be performed. (c) Multiplexed detection of cancer markers: human IgG (black line), PSA (red line), CEA (green line), and AFP (blue line). (d) SEM images of four SiNW-FET sensors incubated in immunogolds conjugated with PSA, CEA, and AFP after the multiplexed electrical measurements [53].
Recently, many emerging SiNW-based biosensors have been designed for in-depth studying in protein detection. Gong et al. [54] performed the ultra-sensitive detection of IgG using the SiNW-FET biosensor. They fabricated a single SiNW-FET, a double SiNW-FET, and a quad SiNW-FET into a single chip and detected the Norovirus DNA and IgG at concentrations of 1 fM and 10 fM, respectively. Then, as shown in Figure 3, Li et al. [55] developed a multichannel dual-gate SiNW-FET chip for dual-channel detection of BC tumor markers. By modifying monoclonal CA15-3 and CEA antibodies on different SiNW surfaces, the SiNW-FET could be used for the dual-channel specific detection of breast cancer tumor markers, CA15-3 and CEA, respectively. The experimental results showed that the double-gated SiNW-FET can amplify the detection signal exponentially through the capacitive coupling effect, thus improving the signal-to-noise ratio of the SiNW-FET. The sensor can accurately detect CA15-3 down to 0.1 U/mL and CEA at 0.01 ng/mL. Hence, compared with the traditional SiNW-FET, the multichannel design method increased the source–drain current signals, effectively restrained the fluctuation noise inside the transistor, and made the detection system more stable.
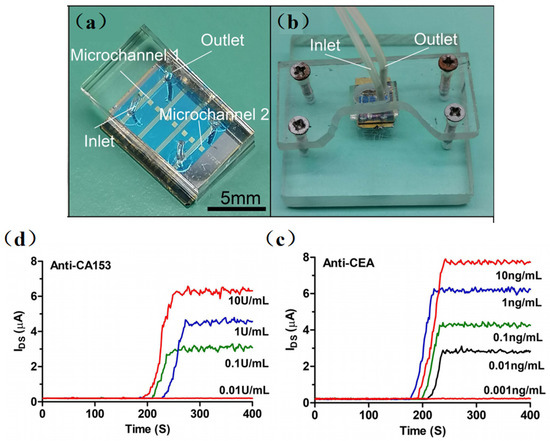
Figure 3. (a) Optical image of the PDMS microfluidic device integrated with the SiNW-FET chip. (b) Optical image of the SiNW-FET biosensor integrated with acrylic fastening fixture. (c) The anti-CA15-3 SiNW-FET biosensor response to different concentrations of CA15-3 in 0.01 × PBS solution. (d) The anti-CEA SiNW-FET biosensor response to different concentrations of CEA in 0.01 × PBS solution. [55].
However, there are performance differences between the SiNW-FET devices manufactured by the same process. Lu et al. developed a novel SiNW biosensor for the detection of human tear MMP-9 based on an optimized fabrication of an optical calibration and low salt concentration scheme, achieving high sensitivity (86.96%) and specificity (90%) in the diagnosis of DED. Their device was manufactured to demonstrate minimal sensor-to-sensor variation, with optical calibration and a low salt concentration protocol allowing consistent response between sensors and sensitive and accurate detection of MMP-9 in human tears with high agreement with ELISA results [56].
SiNW-FET, as a novel kind of biosensor, could be affected by the Debye–Hückel screening effect induced by ions during protein detection [29]. The higher concentration of ions in a protein solution, the shorter the Debye length and the higher the screening efficiency. In other word, the Debye length is inversely proportional to the square root of the ionic strength. In general, because of the electrolytic medium, the interacting electric field exerted from the captured target charges is shielded. Therefore, it is necessary to reduce the Debye–Hückel screening effect when using an SiNW-FET biosensor for protein detection.
The common way to reduce the concentration of ions is by diluting the buffer solution to a low concentration. However, it is still far from effective protein detection. Kim et al. [50] explored the effect of the Debye length on the highly sensitive and label-free detection of cTnI, a biomarker for the diagnosis of acute myocardial infarction. The Debye lengths were calculated for PBS with different ion concentrations (1 × PBS, 0.1 × PBS, 0.01 × PBS3; ionic strength 180, 18, and 1.8 mM, respectively). It was found that the sensitivity increased with the decrease in buffer ion concentration, and the optimal condition was 0.01 × PBS. Therefore, the buffer concentration has a great effect on sensor determination [57,58]. But, extremely low salt concentrations may reduce the activity of proteins, aggravating the difficulty of effective detection.
Chang, S. M et al. [59] reported that the developed SiNW-FET exhibited a lower detection limit of 0.016 ng/mL than previously developed FET devices and showed great potential for future applications in point-of-care (POC) diagnostics. Puppo et al. [60] studied a terminal amnestic-modified SiNW-FET sensor that successfully detected an anti-rabbit antigen in a PBS buffer, further demonstrating the detection of anti-rabbit antigens in a tumor extract solution in the presence of 100,000 non-specific proteins [61,62]. At the same time, Meir developed a dissociative state sensing method that uses temporal differentiation between low-affinity matrix components and high-affinity target molecules as they are dissociated, enabling direct sensing when the biological samples on modifications are rinsed by low ionic strength buffer solutions [63].
It is found that the voltage power spectral density generated by current-biased SiNW-FET is related to 1/f-dependence in the frequency domain. Zheng et al. [64] performed frequency domain detection of a prostate-specific antigen, whose detection sensitivity was 10 times higher than that of time domain detection. This frequency domain measurement method showed the prospect of simple and rapid biomarker screening in medical applications, i.e., in disease diagnosis [60] and drug discovery.
So far, most of the traditional methods for detecting mycobacterium tuberculosis do not meet the requirements of actual TB detection, and the SiNW-FET sensor can solve the appeal problem. Ma [65] developed a fast and sensitive SiNW-FET biosensor for the detection of the Mycobacterium tuberculosis Ag85B protein. The detection limit of MTB Ag85B is as low as 0.01 fg/mL (0.33aM), it has good specificity and stability, and the detection response time is within 30 s. And the detection range is from 1 fg/mL to 100 fg/mL. Compared to other methods, the SiNW-FET biosensor requires only a small sample (7 μL) and can detect the Ag85B protein with a significantly wider dynamic linear range in a shorter time period.
Although the SiNW-FET biosensors were mostly used in laboratory work, they demonstrated high detection sensitivity, specificity, and relativity, which have potential for practical applications such as food safety detection and clinical diagnosis.
3. Microbiological Detection
A microorganism is an organism that can be seen only through a microscope, and they are pervasive in life. In fact, any environment that is devoid of microorganisms is certainly the exception rather than the rule. Microorganisms include bacteria, viruses, and fungi. A pathogenic organism is an organism that is capable of causing diseases in a host (person). So far, there have been many large-scale infection outbreaks caused by pathogenic microorganisms. Examples include swine flu in Mexico in 2009, Ebola in West Africa in 2014, and the plague in Madagascar in 2017. And especially the COVID-19 outbreak, which started in 2020, has caused global infections. The large-scale infection outbreak of these pathogenic microorganisms seriously threatens people’s health and life. So, highly sensitive detection methods are essential for the detection of bacteria and viruses to prevent the spread of infection.
Molecular detection methods are used for the detection of microorganisms. A polymerase chain reaction (PCR) [34] was used in an amplifier to amplify specific DNA fragments, and could be used for bacteria or virus detection with strong specificity, high sensitivity, and low purity requirements. Maan et al. [66] reported a real-time reverse transcription polymerase chain reactions (RT-PCR) assay to recognize Seg-2 of the eight epizootic hemorrhagic disease virus (EHDV) serotypes [67,68]. However, such detectors are difficult to implement for point-of-care testing. An immunofluorescence assay (IFA) could also be used for virus detection. Darwish et al. [69] designed a sensitive and specific optical immunosensor for detecting dengue virus markers using a fluorescent signal label with quantifiable signals [70]. Surface-enhanced Raman Scattering (SERS) [71,72] has emerged as a promising tool to guide cancer diagnosis and synergistic therapy. However, SERS requires the use of nanomaterials to enhance the fluorescence signal, and the detection equipment is relatively expensive. Yin et al. [73] reported an ultra-sensitive, highly selective, and SERS-based SARS-CoV-2 simulation sequence (N-cDNA) detection platform. The platform used a magnetic field to control the distance at which superparamagnetic iron oxide nanoparticles (DNA2/DNA3−SPIONs), DNA2-bound N-cDNA, and AuNPs coupled with DNA3 and their complementary sequence (DNA4) are bound, which effectively enhances the fluorescence signal, thereby increasing its sensitivity.
However, this method is too complicated, time-consuming, and expensive, as is the case with electrochemical biosensors [74]. Currently, the SiNW-FET-based biosensor, as an emerging biological detection technology, could be used for protein [54], DNA [75], and microorganism detection [34], providing new solutions for infectious diseases and other health aspects. To date, SiNW-FETs have successfully detected many viruses, including Dengue [4], Influenza A H3N2 [76], H1N1 [77,78], H5N1 [79], and H5N2 [80]; they have also detected two different viruses simultaneously [81].
To improve the detection sensitivity, researchers improved the SiNW-FET biosensor from different aspects. Patolsky et al. [27] functionalized the nanowire array and observed the changes in electrical conductivity before and after binding in order to detect influenza A labeled with fluorescence. The binding of the viruses resulted in a decrease in the electrical conductivity, which returned to baseline when the viruses were released. Meanwhile, their electrical measurements with antibody-functionalized nanowire field effect transistors (NWFET) illustrated that single viruses can be detected in parallel with high detection selectivity.
Since silanaldehydes are linked to antibodies by reductive amination, it is difficult to reverse the amine link to the original free aldehyde state after antigen detection. Therefore, removing the tightly bound antigen–antibody complex is difficult, resulting in sensors that can only be used once. Chiang et al. [80] proposed a reversible surface modification technology using a disulfide linker, where the receptor molecules were anchored in an MPTMS/SiNW-FET surface with the disulfide bond. Figure 4 shows the experimental setup of the SiNW-FET system, a schematic diagram, and experimental results. The detection limit was 10−17 M for H5N2 avian influenza virus (AIV) allergic testing. The sensing device was reusable by fixing the antibody to the SiNW-FET via disulfide bonds and then reducing the disulfide bonds with dithiothreitol (DTT) while removing the antibody–virus complex. This reversible surface functionalization by reducing disulfide bonds is comparable to another technique that modifies the surface of SiNW-FET by reversible binding–dissociation between glutathione (GSH) and glutathione S-transferase (GST)-labeled proteins [17].
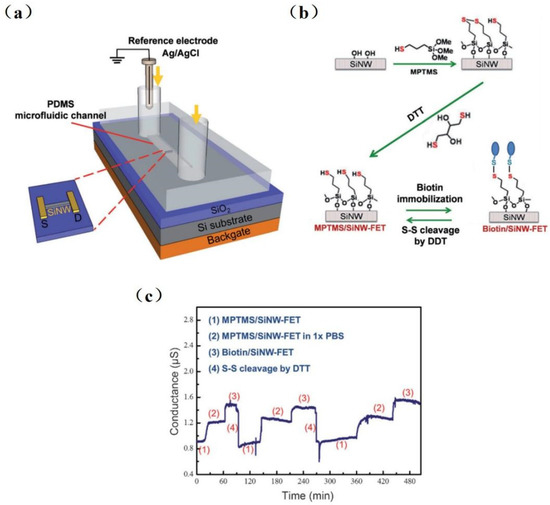
Figure 4. Schematic diagram of the reversible surface functionalization. (a) An experimental setup of SiNW-FET system. (b) The surface of SiNW-FET was modified with MPTMS and then washed with DTT to form an MPTMS/ SiNW-FET. Biotin-HPDP is fixed on MPTMS/SiNW-FET by disulfide bond, and the disulfide linker is subsequently cracked by DTT, and then the device surface returns to the state of MPTMS/SiNW-FET. (c) The reusability of SiNW-FET is proven by three repeatable-period experiments. (1) DTT washes a MPTMS/SiNW-FET, then (2) it is immersed in 1 × PBS and reacts with biotin-HPDP to form (3) biotin/SiNW-FET, and last, the biotin was removed by DTT washing to return [79].
The interaction between hemagglutinin (HA) on Influenza virus’ surface and glycans on the host cell was discussed by Hideshima et al. [23]. They were the first to detect H1 and H5 of influenza A viral HA molecules with glycan-immobilized FET biosensor.
In addition to enhancing surface modification, some improvements were introduced during hardware manufacturing. Borgne et al. [82] developed a simple and low-cost bacteria sensor with a self-assembled gas liquid solid method (VLS). It could be used for label-free and ultra-sensitive bacteria detection. Borgne and his colleagues also developed an SiNW-FET-based resistor fabricated by the VLS method and compatible COMS silicon technology. It was found that Escherichia coli bacteria were able to attach precisely to the SiNW networks, which resulted in a drastic decrease in the electrical resistance of the device [83]. Kim et al. [81] improved the sensitivity of biosensors through material selection. One-dimensional SiNW with a nanoscale three-dimension transistor structure was fabricated through isotropic and anisotropic patterning. This sensor could be used for the multiplex detection of AI and HIV viruses.
SiNW-FET, as an emerging detection method, is applied in the field of biological aerosol research. Shen et al. [76] developed a real-time monitoring system for airborne influenza H3N2, integrating electronic-addressing SiNW sensor devices, microfluidics, and bioaerosol-to-hydrosol air sampling techniques. The experimental results showed that the device can rapidly detect influenza A (H3N2) viruses by alternating clean air samples, indoor air samples, and airborne virus samples with a detection limit of 104 viruses/L and specifically differentiate between H3N2 and H1N1 viruses. The optical image chip and microfluidic channel of SiNWs are shown in Figure 5. This monitoring system was tested with an antenna and network. The remote platforms (such as cell phones and computers) observed continuous conductivity changes in seconds, demonstrating the ability to monitor the influenza virus in real time.
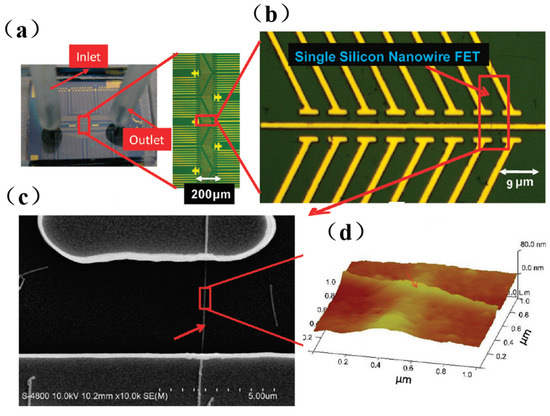
Figure 5. Representative optical images of silicon nanowire (SiNW) chip and microfluidic channel: (a) The microfluidic channel of the SiNW sensor array with one inlet and one outlet. (b) The enlarged portion (red rectangle (A)) of the SiNW sensor device. (c) The SEM image of a single SiNW-FET indicated by arrows in (b). (d) Atomic force microscopy of antibody-modified silicon nanowires [76].
The outbreak of the COVID-19 pandemic started in late December 2019 and then spread quickly worldwide, leading to a global public health emergency. Hu et al. [84] proposed an SiNW-FET platform for the detection of IL-6, a disease marker of COVID-19. By using anti-IL-6 aptamers to detect IL-6, SiNW-FET can achieve effective detection concentrations as low as 2.1 pg/mL (100 fM). Moreover, the proposed aptamer-functionalized SiNW-FET detection range is sufficient to measure IL-6 expression levels in COVID-19-infected patients and distinguish between mild and severe disease.
This entry is adapted from the peer-reviewed paper 10.3390/s23156808
This entry is offline, you can click here to edit this entry!
