Antimicrobial peptides display the property of combating viruses in swine production of animal husbandry, clarify the mechanism of action of antimicrobial peptides on viruses and raise some suspending questions and prospect the future potential of antimicrobial peptides in animal husbandry.
- antimicrobial peptide
- antiviral
- swine
- mechanism of action
Please note: Below is an entry draft based on your previous paper, which is wrirren tightly around the entry title. Since it may not be very comprehensive, we kindly invite you to modify it (both title and content can be replaced) according to your extensive expertise. We believe this entry would be beneficial to generate more views for your work. In addition, no worry about the entry format, we will correct it and add references after the entry is online (you can also send a word file to us, and we will help you with submitting).
Definition
Viral infectious diseases pose a serious threat to animal husbandry, especially in the pig industry. With the rapid, continuous variation of viruses, a series of therapeutic measures, including vaccines, have quickly lost their efficacy, leading to great losses for animal husbandry. It has been reported that antimicrobial peptides (AMPs) have great potential for development and application in animal husbandry because of their significant antibacterial and antiviral activity, and the antiviral ability of AMPs has become a research hotspot.
1. Introduction
Viral infection of pigs is one of the bottlenecks restricting the development of the pig industry globally. The widely spread common porcine pathogenic viruses, including herpesvirus (such as pseudorabies virus (PRV)), coronavirus (such as porcine epidemic diarrhea virus (PEDV)), and arterivirus (such as porcine reproductive and respiratory syndrome virus (PRRSV)), have caused many serious infectious diseases and huge economic losses in the pig industry [1,2,3]. At present, only a few effective treatments are available for most viral diseases. In the past few decades, research on fighting viral infections in pigs has been mainly focused on vaccines, wherein the adaptive immunity of pigs is improved by vaccination [4,5]. However, some viruses can escape from host immunity through different strategies. It is reported that PRRSV evades the host immune response by glycosylation modification of its envelope proteins [6], and PEDV evades the host innate immune response by encoding interferon (IFN) antagonists to disrupt the innate immune pathway and hide its viral RNA to avoid exposure of viral RNA to immune sensors [7]. The emergence of new variants of viruses is one of the important causes of disease outbreaks in pigs, such as the reemergence of PRV in China since late 2011 [8], the outbreak of porcine epidemic diarrhea (PED) in China at the end of 2010 [9], and the spread of African swine fever worldwide since 2007 [10]. Besides, the development of new vaccines is usually complex, technically challenging, and time-consuming [11]. Therefore, there is an urgent need to develop novel effective agents to kill viruses and prevent their infection in swine [4].
Antimicrobial peptides (AMPs) are small proteins with potential activity against, for example, bacteria, viruses, fungi, tumors, and parasites, which are widely found in animals, plants, and microorganisms [12]. The discovery of AMPs can be traced back to 1939; Dubos isolated an antibacterial agent from a strain of soil Bacillus and found that it could protect mice from pneumococcal infection for the first time [13,14]. According to their key structural characteristics, AMPs can be usually divided into four categories: α-helix AMPs, β-folded AMPs, extended structural AMPs, and cyclic structural AMPs [15]. Since their discovery, AMPs have become important alternative drugs in the field of disease prevention and immune regulation, attracting worldwide attention [16,17]. In the field of human viral diseases, at present, AMPs have become an important direction and field in antiviral research. Previous studies have shown that the amphibian-derived AMPs, caerin 1.1 and maculatin 1.1, completely inhibited human immunodeficiency virus (HIV) [18]; arthropod-derived AMPs, cecropin A and melittin, could effectively inhibit Junin virus (JV) multiplication and impede the multiplication of herpes simplex virus (HSV) and JV, respectively [19]; plant protein kalata B1 analogs could inhibit dengue virus (DENV) [20]. The above results suggested that AMPs with antiviral activity have the potential to be antiviral drugs and could be highly expected to become clinical drugs for both human viral diseases and animal viral infections. However, there have only been a few studies on AMPs in animals compared with humans so far. It is important to pay attention to the role of AMPs in animal health because most upstream studies specialized for human usage are run with model animals.
AMPs exhibit different mechanisms on viruses, and their main antiviral mechanisms and types are summarily shown in Figure 1. (i) AMPs neutralize viruses by integrating into the viral envelope or host cell membranes, and both enveloped RNA and DNA viruses can be targeted [21,22]. Indolicidin could inactivate human immunodeficiency virus (HIV) by binding to the envelope and cracking the membrane through a membrane splitting mechanism, thereby preventing the virus from infecting the host cell [23]. (ii) AMPs bind to glycoproteins on the virus surface to inhibit viral infection. Defensin retrocyclin 2 bound to immobilized herpes simplex virus type 2 (HSV-2) glycoprotein B with high affinity so that HSV-2 could not bind to the surface of host cells [24]. (iii) AMPs can interact with specific receptors of host cell membranes, preventing virus particles from binding to host cells. HSV particles infect host cells by binding to heparan sulfate on the host cells, while the α-helix cationic polypeptide lactoferrin can prevent HSV infection by occupying heparan sulfate [25]. (iv) In addition to the above points, AMPs may also act on other stages of the viral life cycle. For example, human beta-defensin-3 (hBD-3) inhibits HIV replication by acting on entry, reverse transcription, and nuclear import of retroviral DNA [26]. Besides, some AMPs, such as cecropin D (CD), could block apoptosis induced by PRRSV at the late stage of infection, thus inhibiting the assembly, release, and transmission of the virus [27].
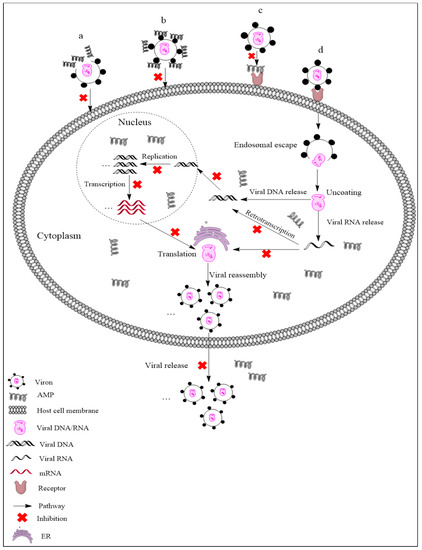
As the pig industry has developed to account for the largest proportion of food animals in China, AMPs have been widely studied as antimicrobial agents and/or feed additives [28,29,30,31]. Studies have shown that the plectasin-derived peptides NZ2114 and MP1102 could effectively kill Streptococcus suis type 2 [28,29], and NZX exhibited antibacterial activity against Staphylococcus hyicus [30]. Diets supplemented with AMPs could improve the growth performance, nutrition maintenance, intestinal morphology, and immunity of weaned piglets, while also reducing the presence of harmful microorganisms in these animals [31]. In addition to antibacterial activity and immune regulation, AMPs should also be actively encouraged and utilized to combat common porcine pathogenic viruses in pig molecular breeding via genetic engineering.
2. Antimicrobial Peptides Used against Viruses in Swine
2.1. AMPs Active against PRV and PEDV
PRV is a large, enveloped, double-stranded DNA virus that is the pathogen of Aujeszky’s disease and belongs to the porcine neurotropic herpesviruses [1,32]. Clinically, it is characterized by severe neurological disorders in newborn piglets and reproductive disorders in sows [32]. PEDV is also an enveloped virus; it is a positive-sense, single-stranded RNA virus, belonging to the family of Coronaviridae [2]. PEDV is the pathogen of porcine epidemic diarrhea (PED), which is an acute infectious enteropathy and is characterized by severe watery diarrhea, vomiting, and dehydration of suckling piglets, causing huge economic losses for the pig industry [33].
2.2. AMPs Active against PRRSV
Porcine reproductive and respiratory syndrome (PRRS), also known as blue-ear pig disease, is one of the most fatal infectious diseases in the pig industry around the world; it was first reported in North America and Canada in the late 1980s [67,68,69]. The pathogen of this disease is called the porcine reproductive and respiratory syndrome virus (PRRSV), which is an enveloped, single-stranded RNA virus belonging to the Arteriviridae family of Nidovirales. Its genomic RNA length is about 15.4 kb, with a 5′ cap, 3′ polyadenylation, and 10 open reading frames (ORFs) [3,70]. The main manifestations of the disease are poor reproductive performance and high miscarriage rate in pregnant sows and dyspnea in growing–finishing pigs and piglets [71]. PRRSV mainly infects porcine alveolar macrophages (PAMs) and has the characteristics of high mutation rate and high recombination rate. With the antigen variation and genetic drift of the virus, the existing vaccines are easily losing their efficacy [72,73]. Therefore, PRRSV is still the greatest challenge facing the pig industry so far, and it is urgent to develop new antiviral strategies to combat PRRSV infection [74].
2.3. Epinecidin-1 (Epi-1) Fights against FMDV
Foot and mouth disease (FMD) is a very important disease affecting livestock in the world [84] and is caused by foot and mouth disease virus (FMDV) [85]. FMDV is a nonenveloped virus belonging to the family of Picornaviridae and genus Aphthovirus [86]; it is highly infectious to pigs and other cloven-hoofed animals and imposes a significant impact on the global economy [87,88]. Common symptoms of foot and mouth disease include fever and blistering lesions in the mouth, tongue, and feet. There are seven antigenic serotypes of FMDV, including O, A, C, SAT1, SAT2, SAT3 (South African 1, 2, 3), and Asia1 (Asian 1); each serotype has multiple subtypes [89], and serotype O is the most common serotype in the world. As there is still no effective vaccine or antiviral drug, new and better drugs or candidates are being sought to combat FMDV infection.
Epinecidin-1 (Epi-1) is derived from the orange-spotted grouper, Epinephelus coioides [90], and belongs to the piscidin peptide family. The piscidin family is an evolutionarily conserved, linear, amphiphilic, antibacterial peptide family that is unique to fish and homologous to cecropins [91]. The length of complete Epi-1 cDNA is 518 base pairs, and the longest open reading frame consists of 204 base pairs and encodes a sequence of 67 amino acids [90]. Studies on the potential pharmacological activity have mainly focused on amino acid residues 22–42 of Epi-1 (Table 1) [53], which shows an α-helical structure without disulfide bonds (Figure 2g) [57]. Epi-1 has been reported to have wide activity against bacteria, fungi, and viruses and shows immune regulation [92,93,94]. Besides, Huang et al. found that the synthetic Epi-1 effectively suppresses FMDV (type O/Taw/97) by inactivating virus particles and inhibiting virus proliferation. Epi-1 not only shows a direct antiviral effect on FMDV at high concentration (10 × EC90 concentration of 125 μg/mL) but also prevents the adsorption of FMDV on BHK-21 cells at low concentration (6.2 μg/mL) [54]. Since a structured membrane is absent in FMDV, research data indicated that the application of Epi-1 in virus adsorption can effectively inhibit virus replication, and thus it is suggested that Epi-1 could interfere with the early stage of viral infection through an undisclosed mechanism [54].
2.4. Synthesized Peptides Fight against ASFV
In addition to the above several viral diseases, African swine fever (ASF) is also a viral disease that lacks effective vaccines for prevention and control in the pig breeding industry; it has spread quickly as an epidemic viral disease in China since mid-2018. As a highly infectious viral disease of pigs, ASF causes fatal hemorrhagic fever after infection, resulting in a high mortality rate of nearly 100% [95]. ASF is caused by African swine fever virus (ASFV), which is a large, enveloped, double-stranded DNA virus with icosahedral morphology and the only member of Asfarviridae family. ASFV is transmitted by arthropod soft ticks (Ornithodoros moubata), making it the only DNA virus to be transmitted via insect [96,97].
Studies have shown that ASFV utilizes dynein for internalization and intracellular transport [98], entering into host cells through dynein- and clathrin-dependent endocytosis and micropinocytosis (Figure 2) [95,99]. As a microtubular motor protein, dynein is in charge of the intracellular transport linked to microtubules. In the early stage of the virus life cycle, the virus carries out intracellular transportation along microtubules. Once the virus passes through the cytoplasm, it quickly enters into the perinuclear region or nucleus and starts to replicate [100]. P54 is the main protein of the ASFV particle membrane, which can interact with the light-chain dynein of 8 kDa (DLC8) both in vitro and in cells. This interaction allows ASFV to be transported to a viral factory located in the perinuclear area at the microtubular organizing center (MTOC), which is necessary for viral protein synthesis and replication [98]. As breaking the interaction between the virus and dynein can hinder the transportation of the virus, it should be focused on as one of the mechanisms of AMPs against ASFV.
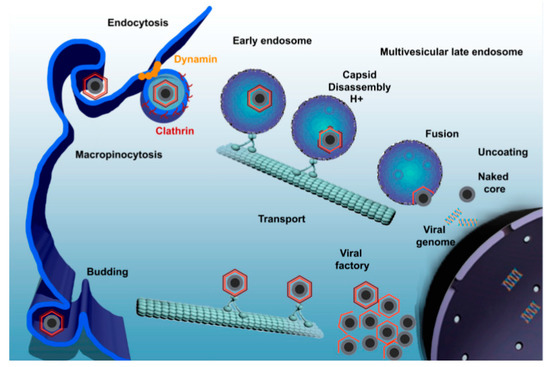
In recent years, synthetic AMPs with specific targets have been designed to bind to receptors on the surface of host cells, rendering these binding sites unavailable to viral proteins and thus impairing viral adsorption [55,101,102]. DNBLK1 is a synthesized short peptide that consists of 28 amino acid residues and contains DLC8 binding domain; by binding to DLC8 to prevent the interaction between ASFV protein p54 and DLC8 in vitro, it may be a useful tool to retard viral replication or spread [55]. Bruno et al. indicated that DNBLK1 can reduce the infectivity, replication, and production of ASFV, and the inhibition occurs at the early stage of the ASFV infection cycle. This provides clues for the treatment of African swine fever and other diseases caused by viruses with the same transmission mechanism as ASFV [55], and more attention should be paid to this new direction.
3. Conclusions
Since there is still no effective treatment for most viral infections in animal husbandry, outbreaks of viral epidemics are generally followed by the quarantine and slaughter of infected animals, resulting in great economic losses for the breeding industry and society [33,68,84,88]. In the past few decades, research on viral diseases in pig breeding has focused on the development of vaccines [5]. Vaccination can inhibit the development of the disease [40], but with the continuous variation of viruses, traditional vaccines lose their effect on mutated virus strains, and the emergence of mutant strains leads to the outbreak of viral diseases. In the context of today’s highly globalized world, viral diseases spread much faster and easier than they did centuries ago [10]. We have to face this threating challenge by utilizing more feasible options.
Therefore, in addition to the usual development of vaccines as antiviral agents, it is also of great significance to exploit new strategies to combat viruses. Currently, AMPs, due to their effective antiviral activity, are the research focus in the field of new antiviral drug development and are expected to become one of the key drivers of antiviral drug development in the future. AMPs inactivate viruses by destroying the viral envelope; binding with virus surface glycoprotein; occupying specific receptors of the host cell membrane; and inhibiting viral replication, transcription, reverse transcription, expression, and release. With the improvement of bioinformatics science, more new AMPs with antiviral activity are continuously being discovered and designed. These AMPs can resist not only porcine viruses but also Newcastle disease virus [103], duck hepatitis virus [104], bovine herpesvirus 1 [105], dengue virus [106], and other human and zoonotic viruses and thus hold great importance for research and development in theory and practice. In addition, synthetic AMPs with specific targets, such as DNBLK1 (targeting the DLC8 binding domain), have also been designed, providing a new idea and tool for the development and improvement of AMPs to resist viruses [55].
Although AMPs have great potential activity against viruses, there are still some potential problems to be solved, such as higher cost of production, shorter half-life time, and poor oral absorption of AMPs, as well as the challenge of delivery systems [107]. Some AMPs have been shown to have antiviral effects in vitro against viral diseases in animal husbandry, but their antiviral activity in vivo remains to be studied and confirmed [27,50,52]. It is known that AMPs are sensitive to trypsin and other lytic factors in vivo, especially when they are administrated orally or by blood injection. In order to improve the stability of AMPs, many studies have been carried out considering the controlled site-specific release and sustained continuous release of AMPs by nanoencapsulation [108,109] and modification of high resistance to proteolysis [110], as well including unnatural or D-amino acids substitution [111] and peptide chain cyclization [112,113]. Moreover, targeting modification is also worth considering as a powerful tool to increase killing specificity to pathogens and decrease host cell toxicity of AMPs [114,115]. In addition, many mechanisms of action between AMPs and virus molecules are still unclear and need to be further studied. However, we find it reasonable to assume that the previous fruitful findings and constructive theories from antibacterial studies with AMPs in vivo and in vitro, such as those concerning the mechanism of entry into the host cell and bactericidal details, might be shared and referenced during antiviral studies; it is confirmed from previous works that AMPs enter the blood circulation through different drug delivery routes, reach various organs, and further internalize into the cells through endocytosis and micropinocytosis [116,117,118,119]. Undoubtedly, wider and deeper new findings are highly deserving of anticipation and will attract great interest due to the unique advantages of AMPs, including their high penetration into the host cell owing to their intracellular origin, their close compatibility with the host, their hypersensitive early-warning/protection response to infection, and their low drug resistance rate owing to strong penetration and multitargeting of pathogens [30,120,121,122,123,124,125]. We strongly believe and optimistically expect that with the further elucidation of the structure, expression regulation, and mechanism of action of AMPs as a whole, the factors that limit the development of AMPs will be disclosed and overcome one by one, and more new functions of AMPs will be discovered. Eventually, AMPs will be widely utilized and commercialized in animal husbandry and even in the human health industry [16,124,126].
In summary, antimicrobial peptides, which can effectively combat viruses, are highly expected to break through their special technical bottleneck in the near future; this will enable them to support the sustainable green development of the husbandry and health industries and thus promote the general upgrading of green industry in China and the world.
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics9110801
