The first cases of COVID-19, which is caused by the SARS-CoV-2, were reported in December 2019. The vertiginous worldwide expansion of SARS-CoV-2 caused the collapse of health systems in several countries due to the high severity of the COVID-19. In addition to the vaccines, the search for active compounds capable of preventing and/or fighting the infection has been the main direction of research. Since the beginning of this pandemic, some evidence has highlighted the importance of a phenolic-rich diet as a strategy to reduce the progression of this disease, including the severity of the symptoms. Some of these compounds (e.g., curcumin, gallic acid or quercetin) already showed capacity to limit the infection of viruses by inhibiting entry into the cell through its binding to protein Spike, regulating the expression of angiotensin-converting enzyme 2, disrupting the replication in cells by inhibition of viral proteases, and/or suppressing and modulating the host’s immune response.
- COVID-19
- immune response
- phenolic compounds
1. Introduction
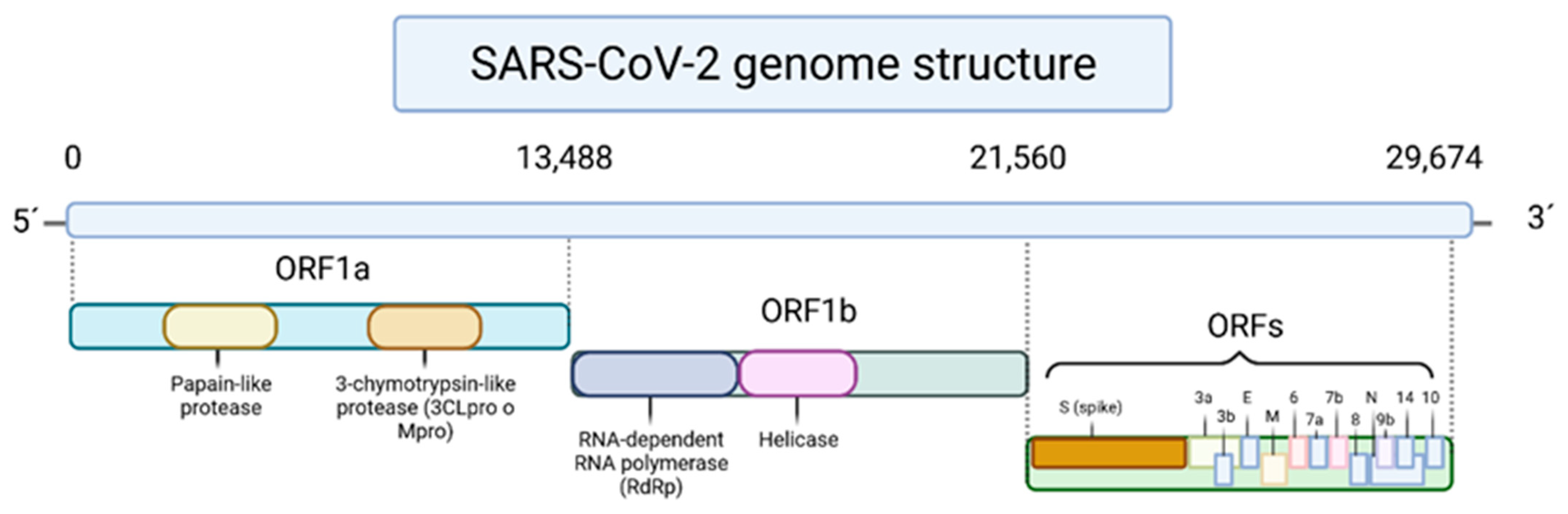
2. Phenolic Compounds in Human Health
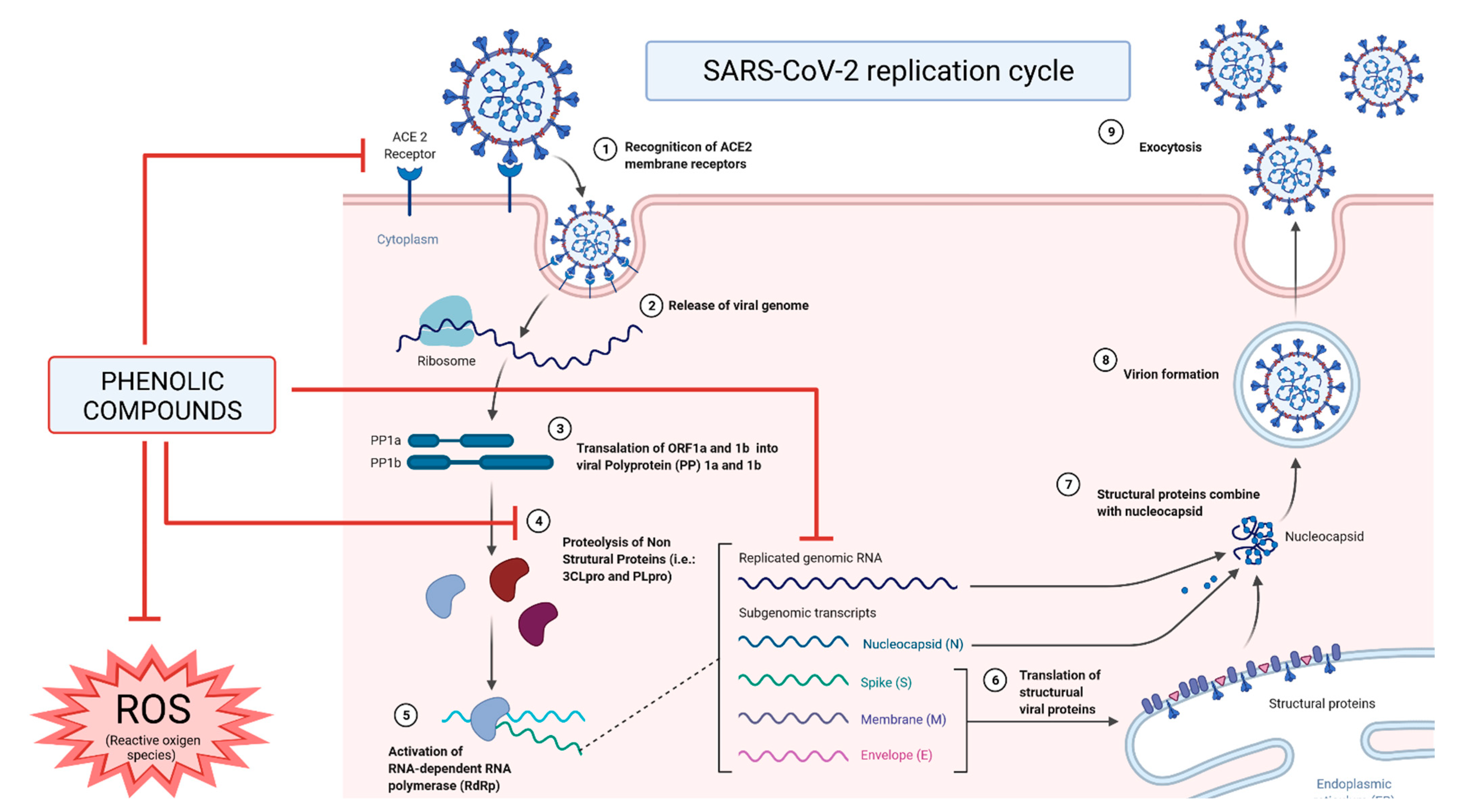
3. Phenolic Compounds in COVID-19
|
Phenolic Compounds |
Main Outcome |
Reference |
|---|---|---|
|
Cyanidin |
Mpro inhibitor |
|
|
Daidzein |
||
|
Dieckol |
||
|
Genistein |
||
|
Mearnsitrin |
||
|
Myricitrin |
||
|
Psoralidin |
||
|
Quercetin 3-O-β-D-glucoside |
||
|
Rutin |
||
|
Xanthoangelol E |
||
|
Benzoic acid |
RdRp inhibitor |
|
|
Cyanidin |
||
|
Daidzein |
||
|
Ellagic acid |
||
|
Gallic acid |
||
|
Genistein |
||
|
Kaempferol 3-O-rutinoside |
||
|
Naringenin |
||
|
Oleuropein |
||
|
Quercetin |
||
|
Quercetin 3-O-rutinoside |
||
|
Resveratrol |
||
|
Myrcetin |
Non-structural SARS-CoV-2 helicases inhibitor |
[68] |
|
Scutellarein |
||
|
Cyanidin 3-O-glucoside |
PLpro inhibitor |
|
|
Epigallocatechin |
||
|
Epigallocatechin gallate |
||
|
Hypericin |
||
|
Kaempferol |
||
|
Quercetin |
||
|
Cryptotanshinone |
TMPRSS2 inhibitor |
|
|
Ellagic acid |
||
|
Gallic acid |
||
|
Kaempferol |
||
|
Luteolin |
||
|
Quercetin |
||
|
Afzelin |
ACE2 inhibitor |
|
|
Apigenin |
||
|
Baicalin |
||
|
Biorobin |
||
|
Caffeic acid |
||
|
Catechin |
||
|
Chlorogenic acid |
||
|
Chrysin |
||
|
Ellagic acid |
||
|
Curcumin |
||
|
Cyanidin |
||
|
Delphinidin |
||
|
Epigallocatechin |
||
|
Epigallocatechin gallate |
||
|
Ferulic acid |
||
|
Galangin |
||
|
Gallic acid |
||
|
Hesperetin |
||
|
Isoferulic acid |
||
|
Kaempferol |
||
|
Luteolin |
||
|
Myricitrin |
||
|
Naringenin |
||
|
Nobiletin |
||
|
Nympholide A |
||
|
Pinocembrin |
||
|
Quercetin |
||
|
Rhoifolin |
||
|
Rutin |
||
|
Scutellarein |
||
|
Taiwanhomoflavone A |
||
|
Tangeretin |
||
|
ε-Viniferin |
||
|
Chrysin |
Interact with Spike protein |
|
|
Ellagic acid |
||
|
Gallic acid |
||
|
Hesperetin |
||
|
Pinocembrin |
||
|
Artepillin C |
Inhibit p21-activated kinase 1 |
[74] |
|
Ellagic acid |
Inhibit furin |
[75] |
|
Gallic acid |
Abbreviations: Mpro, Main Protease; PLpro, papain-like protease; RdRp, RNA-dependent RNA polymerase; TMPRSS2, transmembrane protease serine 2; ACE2, Angiotensin-Converting Enzyme II.
This entry is adapted from the peer-reviewed paper 10.3390/foods10092084
References
- Jiang, S.; Du, L.; Shi, Z. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: Calling for developing therapeutic and prophylactic strategies. Emerg. Microbes Infect. 2020, 9, 275–277.
- Lytras, S.; Hughes, J.; Xia, W.; Jiang, X.; Robertson, D.L. Exploring the natural origins of SARS-CoV-2. bioRxiv. 2021. Available online: https://www.biorxiv.org/content/10.1101/2021.01.22.427830v3.abstract (accessed on 27 June 2021).
- Sallard, E.; Halloy, J.; Casane, D.; Decroly, E.; van Helden, J. Tracing the origins of SARS-COV-2 in coronavirus phylogenies: A review. Environ. Chem. Lett. 2021, 19, 769–785.
- Wacharapluesadee, S.; Tan, C.W.; Maneeorn, P.; Duengkae, P.; Zhu, F.; Joyjinda, Y.; Kaewpom, T.; Chia, W.N.; Ampoot, W.; Lim, B.L.; et al. Evidence for SARS-CoV-2 related coronaviruses circulating in bats and pangolins in Southeast Asia. Nat. Commun. 2021, 12, 1–9.
- Makarenkov, V.; Mazoure, B.; Rabusseau, G.; Legendre, P. Horizontal gene transfer and recombination analysis of SARS-CoV-2 genes helps discover its close relatives and shed light on its origin. BMC Ecol. Evol. 2021, 21, 1–18.
- Boopathi, S.; Poma, A.B.; Kolandaivel, P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J. Biomol. Struct. Dyn. 2020, 1–10.
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273.
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 1–10.
- Sriram, K.; Insel, P. A hypothesis for pathobiology and treatment of COVID-19: The centrality of ACE1/ACE2 imbalance. Authorea 2020, 177, 4825–4844.
- Singh, A.; Gautam, A.; Chandel, S.; Ghosh, A.; Dey, D.; Roy, S.; Ravichandiran, V.; Gosh, D. Protease inhibitory effect of natural polyphenolic compounds on SARS-CoV-2: An in silico study. Molecules 2020, 25, 4604.
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1–42.
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246.
- Monagas, M.; Bartolomé, B.; Gómez-Cordovés, C. Updated Knowledge About the Presence of Phenolic Compounds in Wine. Crit. Rev. Food Sci. Nutr. 2005, 45, 85–118.
- Jesus, F.; Gonçalves, A.C.A.; Alves, G.; Silva, L.R. Health Benefits of Prunus avium Plant Parts: An Unexplored Source Rich in Phenolic Compounds. Food Rev. Int. 2020, 1–29.
- Calumpang, C.L.; Saigo, T.; Watanabe, M.; Tohge, T. Cross-Species Comparison of Fruit-Metabolomics to Elucidate Metabolic Regulation of Fruit Polyphenolics Among Solanaceous Crops. Metabolites 2020, 10, 209.
- Quiles, J.L.; Rivas-García, L.; Varela-López, A.; Llopis, J.; Battino, M.; Sánchez-González, C. Do nutrients and other bioactive molecules from foods have anything to say in the treatment against COVID-19? Environ. Res. 2020, 191, 110053.
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273.
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and flavanones are more bioavailable than previously perceived: A review of recent rvidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180.
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S.
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free. Radic. Biol. Med. 2015, 89, 758–769.
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human intervention study to investigate the intestinal accessibility and bioavailability of anthocyanins from bilberries. Food Chem. 2017, 231, 275–286.
- Halliwell, B. Dietary polyphenols: Good, bad, or indifferent for your health? Cardiovasc. Res. 2007, 73, 341–347.
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326.
- Singh, M.; Thrimawithana, T.; Shukla, R.; Adhikari, B. Managing obesity through natural polyphenols: A review. Futur. Foods 2020, 1–2, 100002.
- Anhê, F.F.; Desjardins, Y.; Pilon, G.; Dudonné, S.; Genovese, M.I.; Lajolo, F.M.; Marette, A. Polyphenols and type 2 diabetes: A prospective review. Pharma Nutr. 2013, 1, 105–114.
- Chait, Y.A.; Gunenc, A.; Bendali, F.; Hosseinian, F. Simulated gastrointestinal digestion and in vitro colonic fermentation of carob polyphenols: Bioaccessibility and bioactivity. LWT 2020, 117, 108623.
- Del Mondo, A.; Smerilli, A.; Ambrosino, L.; Albini, A.; Noonan, D.M.; Sansone, C.; Brunet, C. Insights into phenolic compounds from microalgae: Structural variety and complex beneficial activities from health to nutraceutics. Crit. Rev. Biotechnol. 2021, 41, 155–171.
- Pap, N.; Fidelis, M.; Azevedo, L.; Do, C.M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry polyphenols and human health: Evidence of antioxidant, anti-inflammatory, microbiota modulation, and cell-protecting effects. Curr. Opin. Food Sci. 2021, 42, 167–186.
- Tomé-Carneiro, J.; Visioli, F. Polyphenol-based nutraceuticals for the prevention and treatment of cardiovascular disease: Review of human evidence. Phytomedicine 2016, 23, 1145–1174.
- Lorenz, M.; Lehmann, S.; Djordjevic, I.; Düsterhöft, T.; Zimmermann, B.F.; Stangl, K.; Stangl, V. Vasodilation of Tea Polyphenols Ex Vivo Is Mediated by Hydrogen Peroxide under Rapid Compound Decay. Antioxidants 2020, 9, 390.
- Markovics, A.; Biró, A.; Kun-Nemes, A.; Fazekas, M.É.; Rácz, A.A.; Paholcsek, M.; Lukács, J.; Stündl, L.; Remenyik, J. Effect of Anthocyanin-Rich Extract of Sour Cherry for Hyperglycemia-Induced Inflammatory Response and Impaired Endothelium-Dependent Vasodilation. Nutrients 2020, 12, 3373.
- Sanches-Silva, A.; Testai, L.; Nabavi, S.F.; Battino, M.; Devi, K.P.; Tejada, S.; Sureda, A.; Xu, S.; Yousefi, B.; Majidinia, M.; et al. Therapeutic potential of polyphenols in cardiovascular diseases: Regulation of mTOR signaling pathway. Pharmacol. Res. 2020, 152, 104626.
- Ávila-Román, J.; Soliz-Rueda, J.R.; Bravo, F.I.; Aragonès, G.; Suárez, M.; Arola-Arnal, A.; Mulero, M.; Salvadó, M.J.; Arola, L.; Torres-Fuentes, C.; et al. Phenolic compounds and biological rhythms: Who takes the lead? Trends Food Sci. Technol. 2021, 113, 77–85.
- Olszewska, M.A.; Gędas, A.; Simões, M. Antimicrobial polyphenol-rich extracts: Applications and limitations in the food industry. Food Res. Int. 2020, 134, 109214.
- Loaiza-Cano, V.; Monsalve-Escudero, L.M.; Filho, C.D.S.M.B.; Martinez-Gutierrez, M.; de Sousa, D.P. Antiviral Role of Phenolic Compounds against Dengue Virus: A Review. Biomolecules 2020, 11, 11.
- El-Aziz, N.; Shehata, M.; Awad, O.; El-Sohaimy, S. Inhibition of COVID-19 RNA-dependent RNA polymerase by natural bioactive compounds: Molecular docking analysis. Egypt. J. Chem. 2021, 64, 1989–2001.
- Ogawa, M.; Shimojima, M.; Saijo, M.; Fukasawa, M. Several catechins and flavonols from green tea inhibit severe fever with thrombocytopenia syndrome virus infection in vitro. J. Infect. Chemother. 2021, 27, 32–39.
- Moradi, M.-T.; Karimi, A.; Lorigooini, Z.; Pourgheysari, B.; Alidadi, S. In vitro anti influenza virus activity, antioxidant potential and total phenolic content of twelve Iranian medicinal plants. Marmara Pharm. J. 2017, 21, 843–851.
- Moradi, M.-T.; Karimi, A.; Shahrani, M.; Hashemi, L.; Ghaffari-Goosheh, M.-S. Anti-Influenza Virus Activity and Phenolic Content of Pomegranate (Punica granatum L.) Peel Extract and Fractions. Avicenna J. Med. Biotech. 2020, 11, 285–291.
- Xie, Y.; Huang, B.; Yu, K.; Shi, F.; Liu, T.; Xu, W. Caffeic acid derivatives: A new type of influenza neuraminidase inhibitors. Bioorganic Med. Chem. Lett. 2013, 23, 3556–3560.
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; López-Sabater, M.C.; Covas, M.I.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; et al. Predimed study investigators. Polyphenol intake and mortality risk: A re-analysis of the Predimed trial. BMC Med. 2014, 12, 77.
- Guler, H.I.; Sal, F.A.; Can, Z.; Kara, Y.; Yildiz, O.; Belduz, O.; Çanakci, S.; Kolayli, S. Targeting CoV-2 Spike RBD and ACE-2 Interaction with flavonoids of Anatolian propolis by in silico and in vitro studies in terms of possible COVID-19 therapeutics. Turkish J. Biol. 2021, 45, 530–548.
- Hamed, A.N.; Samy, M.N.; Mahmoud, B.K.; Shihata, E.Z.A.; Ali, T.F.S.; Afifi, A.; Kamel, M.S. Flavonoidal glycosides and in vitro antioxidant activity of Bignonia binata Thunb. leaves Family Bignoniaceae and in silico evidence of their potential anti-COVID-19 activity. J. Adv. Biomed. Pharm. Sci. 2021, 4, 98–106.
- Gowrishankar, S.; Muthumanickam, S.; Kamaladevi, A.; Karthika, C.; Jothi, R.; Boomi, P.; Maniazhagu, D.; Pandian, S.K. Promising phytochemicals of traditional Indian herbal steam inhalation therapy to combat COVID-19—An in silico study. Food Chem. Toxicol. 2021, 148, 111966.
- Singh, R.; Singh, P.K.; Kumar, R.; Kabir, T.; Kamal, M.A.; Rauf, A.; Albadrani, G.M.; Sayed, A.A.; Mousa, S.A.; Abdel-Daim, M.M.; et al. Multi-Omics Approach in the Identification of Potential Therapeutic Biomolecule for COVID-19. Front. Pharmacol. 2021, 12, 652335.
- Limanaqi, F.; Busceti, C.L.; Biagioni, F.; Lazzeri, G.; Forte, M.; Schiavon, S.; Sciarretta, S.; Frati, G.; Fornai, F. Cell Clearing Systems as Targets of Polyphenols in Viral Infections: Potential Implications for COVID-19 Pathogenesis. Antioxidants 2020, 9, 1105.
- Lin, C.-W.; Tsai, F.-J.; Tsai, C.-H.; Lai, C.-C.; Wan, L.; Ho, T.-Y.; Hsieh, C.-C.; Chao, P.-D.L. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir. Res. 2005, 68, 36–42.
- Junior, J.A.C.N.; Santos, A.M.; Quintans-Júnior, L.J.; Walker, C.I.B.; Borges, L.P.; Serafini, M.R. SARS, MERS and SARS-CoV-2 (COVID-19) treatment: A patent review. Expert Opin. Ther. Pat. 2020, 30, 567–579.
- Zabetakis, I.; Lordan, R.; Norton, C.; Tsoupras, A. COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients 2020, 12, 1466.
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 1–7.
- Giovinazzo, G.; Gerardi, C.; Uberti-Foppa, C.; Lopalco, L. Can Natural Polyphenols Help in Reducing Cytokine Storm in COVID-19 Patients? Molecules 2020, 25, 5888.
- Margină, D.; Ungurianu, A.; Purdel, C.; Nițulescu, G.M.; Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Burykina, T.I.; Tekos, F.; Buha, A.; et al. Analysis of the intricate effects of polyunsaturated fatty acids and polyphenols on inflammatory pathways in health and disease. Food Chem. Toxicol. 2020, 143, 111558.
- Tallei, T.E.; Tumilaar, S.G.; Niode, N.J.; Kepel, B.J.; Idroes, R.; Effendi, Y.; Sakib, S.A.; Emran, T. Bin potential of plant bioactive compounds as SARS-CoV-2 main protease (Mpro) and Spike (S) glycoprotein inhibitors: A molecular docking study. Scientifica 2020, 2020, 6307457.
- Mishra, C.B.; Pandey, P.; Sharma, R.D.; Malik, M.Z.; Mongre, R.K.; Lynn, A.M.; Prasad, R.; Jeon, R.; Prakash, A. Identifying the natural polyphenol catechin as a multi-targeted agent against SARS-CoV-2 for the plausible therapy of COVID-19: An integrated computational approach. Brief. Bioinform. 2021, 22, 1346–1360.
- Pitsillou, E.; Liang, J.; Karagiannis, C.; Ververis, K.; Darmawan, K.K.; Ng, K.; Hung, A.; Karagiannis, T.C. Interaction of small molecules with the SARS-CoV-2 main protease in silico and in vitro validation of potential lead compounds using an enzyme-linked immunosorbent assay. Comput. Biol. Chem. 2020, 89, 107408.
- Elfiky, A.A. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: An in silico perspective. J. Biomol. Struct. Dyn. 2020, 39, 3204–3212.
- Kokic, G.; Hillen, H.S.; Tegunov, D.; Dienemann, C.; Seitz, F.; Schmitzova, J.; Farnung, L.; Siewert, A.; Höbartner, C.; Cramer, P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021, 12, 1–7.
- Singh, S.; Sk, F.; Sonawane, A.; Kar, P.; Sadhukhan, S.; Sk, M.F.; Sonawane, A.; Kar, P.; Sadhukhan, S. Plant-derived natural polyphenols as potential antiviral drugs against SARS-CoV-2 via RNA—Dependent RNA polymerase (RdRp) inhibition: An in-silico analysis. J. Biomol. Struct. Dyn. 2020, 28, 1–16.
- Karkhanei, B.; Ghane, E.T.; Mehri, F. Evaluation of oxidative stress level: Total antioxidant capacity, total oxidant status and glutathione activity in patients with COVID-19. New Microbes New Infect. 2021, 42, 100897.
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-Boosting, Antioxidant and Anti-inflammatory Food Supplements Targeting Pathogenesis of COVID-19. Front. Immunol. 2020, 11, 570122.
- Iddir, M.; Brito, A.; Dingeo, G.; Del Campo, S.S.F.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562.
- Abd El-Mordy, F.M.; El-Hamouly, M.M.; Ibrahim, M.T.; El-Rheem, G.A.; Aly, O.M.; Abd El-Kader, A.M.; Youssif, K.A.; Abdelmohsen, U.R. Inhibition of SARS-CoV-2 main protease by phenolic compounds from Manilkara hexandra (Roxb.) Dubard assisted by metabolite profiling and in silico virtual screening. RSC Adv. 2020, 10, 32148–32155.
- Pendyala, B.; Patras, A. In silico screening of food bioactive compounds to predict potential inhibitors of COVID-19 main protease (Mpro) and RNA-dependent RNA polymerase (RdRp). ChemRxiv. 2020. Available online: https://chemrxiv.org/engage/chemrxiv/article-details/60c749a3567dfef273ec4c31 (accessed on 15 June 2021).
- Kumar Verma, A.; Kumar, V.; Singh, S.; Goswami, B.C.; Camps, I.; Sekar, A.; Yoon, S.; Lee, K.W. Repurposing potential of Ayurvedic medicinal plants derived active principles against SARS-CoV-2 associated target proteins revealed by molecular docking, molecular dynamics and MM-PBSA studies. Biomed. Pharmacother. 2021, 137, 111356.
- Cherrak, S.A.; Merzouk, H.; Mokhtari-Soulimane, N. Potential bioactive glycosylated flavonoids as SARS-CoV-2 main protease inhibitors: A molecular docking and simulation studies. PLoS ONE 2020, 15, e0240653.
- Xu, J.; Gao, L.; Liang, H.; Chen, S.-D. In silico screening of potential anti–COVID-19 bioactive natural constituents from food sources by molecular docking. Nutrition 2021, 82, 111049.
- Silva, F.M.A.; Silva, K.P.A.; Oliveira, L.P.M.; Costa, E.V.; Koolen, H.H.F.; Pinheiro, M.L.B.; Souza, A.Q.L.; Souza, A.D.L. Flavonoid glycosides and their putative human metabolites as potential inhibitors of the SARS-CoV-2 main protease (Mpro) and RNA-dependent RNA polymerase (RDRP). Mem. Inst. Oswaldo Cruz 2020, 115, 1–8.
- Benarba, B.; Pandiella, A. Medicinal Plants as Sources of Active Molecules Against COVID-19. Front. Pharmacol. 2020, 11, 1189.
- Swargiary, A.; Mahmud, S.; Saleh, M.A. Screening of phytochemicals as potent inhibitor of 3-chymotrypsin and papain-like proteases of SARS-CoV2: An in silico approach to combat COVID-19. J. Biomol. Struct. Dyn. 2020. Available online: https://www.tandfonline.com/doi/ref/10.1080/07391102.2020.1835729?scroll=top (accessed on 10 June 2021).
- Piccolella, S.; Crescente, G.; Faramarzi, S.; Formato, M.; Pecoraro, M.T.; Pacifico, S. Polyphenols vs. Coronaviruses: How Far Has Research Moved Forward? Molecules 2020, 25, 4103.
- Suručić, R.; Tubić, B.; Stojiljković, M.P.; Djuric, D.M.; Travar, M.; Grabež, M.; Šavikin, K.; Škrbić, R. Computational study of pomegranate peel extract polyphenols as potential inhibitors of SARS-CoV-2 virus internalization. Mol. Cell. Biochem. 2021, 476, 1179–1193.
- Muchtaridi, M.; Fauzi, M.; Ikram, N.K.K.; Gazzali, A.M.; Wahab, H.A. Natural flavonoids as potential angiotensin-converting enzyme 2 inhibitors for Anti-SARS-CoV-2. Molecules 2020, 25, 3980.
- Chen, H.; Du, Q. Potential natural compounds for preventing 2019-nCoV infection. Preprints 2020, 2020, 358.
- Shahinozzaman, M.; Basak, B.; Emran, R.; Rozario, P.; Obanda, D.N. Artepillin C: A comprehensive review of its chemistry, bioavailability, and pharmacological properties. Fitoterapia 2020, 147, 104775.
- Wu, C.; Zheng, M.; Yang, Y.; Gu, X.; Yang, K.; Li, M.; Liu, Y.; Zhang, Q.; Zhang, P.; Wang, Y.; et al. Furin: A Potential Therapeutic Target for COVID-19. Iscience 2020, 23, 101642.
