1. Treatment of Non-Scarring Alopecia
Conventionally, AGA treatments aim at lowering the levels of dihydroxy testosterone by using 5 alpha-reductase inhibitors, minoxidil and finasteride, a potassium channel opener [
26]. Minoxidil (topical 5% solution/foam) and finasteride (oral 1 mg/day) are the first-line therapies for AGA.
Finasteride is an inhibitor of 5 alpha-reductase type 2, which decreases the concentration of DHT in serum and scalp and, hence, increases hair growth. It has to be taken for at least 1 year to notice hair growth and has to be continued to maintain the hair growth. The effect of regrowth will be lost if the drug is stopped for 6–9 months. Erectile and ejaculatory dysfunction are among the many negative effects of finasteride. Gynecomastia, testicular pain, and depression are the rare adverse effects of finasteride [
27,
28]. Finasteride may interfere with the levels of prostatic specific antigen (PSA), it is shown that PSA level decreases considerably in patients taking finasteride [
29].
Minoxidil is a potassium channel opener. It is a vasodilator and induces vascular endothelial growth factor (VEGF) to increase vascularity and dermal papilla size [
30,
31]. The response to treatment is variable. It requires 12–18 months to assess the efficacy of minoxidil. It has to be continued life long and the effect ceases with the stoppage of treatment. There might be exaggerated hair fall in the initial 2 months (telogen to anagen transition phase), but it improves over 2 months. Adverse effects include contact dermatitis, irritant dermatitis, and hypertrichosis over the face [
32,
33]. Other new modalities include platelet-rich plasma (PRP), mesotherapy, lasers, micro-needling of the scalp, and Janus kinase (JAK) inhibitors. When patients are keen on immediate results, they resort to hair transplantation, i.e., follicular unit transplantation (FUT) or follicular unit extraction (FUE).
It is clear from the studies that first-line therapies for AGA that:
1. They have to be continued lifelong, which decreases the compliance;
2. The regrowth ceases with the discontinuation of therapy;
3. They are associated with several adverse effects leading to temporary morbidity.
Alopecia areata (AA) is a non-scarring chronic, immune-inflammatory disorder of hair follicles. The most typical manifestation of AA is the presence of localized patches of hair loss on the scalp, but severe cases can result in generalized hair loss throughout the body [
34]. AA affects about 2% of the general population at some point in life [
35]. The disruption of the hair follicle’s immune privilege is regarded to be a key element in the pathophysiology of AA. It is centered on a lymphocytic infiltration in and around the hair follicle’s bulb or lower part. Despite various therapeutic options, such as corticosteroids (topical, intralesional, oral), tacrolimus, minoxidil, contact immunotherapies like squaric acid dibutyl ester, diphencyprone, and photo(chemo)therapy using UVA and psoralens, there is no cure for AA [
36].
It is obvious that the therapies for AA:
1. Have unpredictable outcome;
2. Have no permanent cure;
3. Are associated with significant adverse reactions.
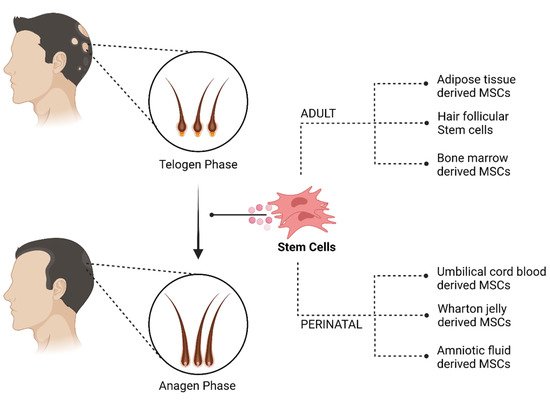
This has led researchers to explore regenerative therapies for hair restoration in non-scarring alopecia. Being autologous/allogenic, they are free from adverse effects with good patient compliance. The regenerative modalities explored are PRP, amniotic fluid, adipose-derived stem cells, follicular micrograft, bone marrow cells, cord blood and Wharton jelly.
Regenerative therapies in non-scarring alopecia can include cells, which can produce factors inducing hair growth or the products of the cells which can be isolated and used. Cells as such are difficult to maintain in culture for transplantation whereas growth-factors secreted by the cells in the medium are easy to transport and is less expensive compared to cellular therapy as such. Hence, it is essential to classify the regenerative therapies into growth factor-rich and stem-cell rich, which will aid in better understanding, clinical utility and for further research. Therefore, researchers suggest this “A La Mode
Table 1. “A La Mode Classification” of regenerative therapies [
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60].
| Stem Cell-Rich |
Growth Factor-Rich |
|
I. Adult stem cells
A. Adipose derived stem cell (ADSC)
1. Nanofat
2. Stromal vascular fraction (SVF)
B. Hair follicular stem cell (HFSCs)
1. Autologous micro grafts [Human intra and extra dermal adipose tissue derived hair follicle stem cells (HD-AFSCs)]
2. Cultured HFSCs
i. Hair follicle-derived MSCs (HF-MSC)
ii. Hair follicle epidermal stem cells (HF-ESC)
C. Bone marrow derived
1. Bone marrow mononuclear cells (BMMC)
2. Bone marrow aspirate concentrate (BMAC)
II. Perinatal stem cells
A. Umbilical cord blood derived
B. Wharton jelly MSCs (WJ-MSC)/Umbilical cord MSCs (UC-MSC)
1. Sub amniotic.
2. Perivascular.
3. Intervascular.
C. Amniotic fluid derived
D. Placental MSC
|
I. Platelet-rich plasma (PRP)
A. Autologous activated PRP (AA-PRP)
B. Autologous non-activated PRP (A-PRP)
II. Autologous growth factor concentrate (GFC)
III. Conditioned medium (Secretomes)
A. ADSC-CM (AAPE)
B. hUCB-MSC-CM
C. AF-MSC-CM
D. HF-MSC-CM
E. BM-MSC-CM (Genetically engineered)
IV. Extracellular vesicles
A. Exosomes
i. New-born foreskin stem cell
ii. DPC
iii. BM-MSC
B. Exosome-like (Ginseng)
C. Microvesicles
V. Placental extract
|
The cellular therapies used to treat non-scarring alopecia, as shown in Figure 1, their mechanism of action and the recent studies to validate its efficacy. In addition, studies on conditioned medium are briefly mentioned at places to reiterate the potential of cellular therapy.
Figure 1. Cellular therapy for non-scarring alopecia. Created with BioRender.com (accessed on 1 March 2022).
2. Cellular Therapy
2.1. Adult Stem Cells
MSCs are the most dynamic, immature, diverse, and multipotent stromal progenitor cells. They have a morphology similar to fibroblasts and have the capacity for trans-differentiation into a range of tissues of ectoderm, endoderm, and mesodermal origin. MSC clonal heterogeneity is demonstrated in terms of variable differentiation, regeneration and proliferative capability in both in vivo and in vitro studies. MSCs express non-differentiating cell surface markers, such as CD146 or CD200. MSCs are found in bone marrow, the placenta, the umbilical cord, fat, menstrual blood, molar teeth and amniotic fluid [
61]. As there are multiple sources of MSCs, it is of immense importance to define an MSC. In 2006, Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy has proposed three minimum criteria to define an MSC. They are as follows:
1. MSC must be plastic adherent when maintained under standard culture conditions.
2. Expression of CD105, CD73 and CD90, and lack expression of CD45, CD34, CD14, or CD11b, CD79a, or CD19 and HLA-DR surface molecules.
3. They must differentiate to osteoblasts, adipocytes and chondroblasts in vitro [
62].
MSCs are more adaptable in nature, allowing them to switch from one differentiation path to another as influenced by growth factors, cytokines, and chemokines. The conversion of chondrocytes into osteoblasts and osteoblasts into chondrocytes has been demonstrated in the literature (trans differentiation) [
63]. Pathway conversion from one cell lineage and another cell lineage can explain the concept of de-differentiation. De-differentiation lineage conversion is based on a phase space model or a noise-driven stem cell differentiation model. Self-renewal and specialization characteristics are inversely proportional. A cell’s plasticity eliminates the necessity for cells to have a constant capability for self-renewal. Plasticity necessitates a cell’s whole differentiation potential in order to form the final product. Lineage priming is another feature of MSCs. The pathway is primed to follow a certain lineage to differentiate into a final cell of interest as a result of the action of growth factors and transcription factors.
The application of stem cells at the site of action entails advocating in two methodical approaches are either direct delivery to target site or systemic administration. Direct delivery implies that harvested stem cells from any source either bone marrow or adipose tissue or placenta or umbilical cord can be injected or implanted at the exact site for regeneration. The procedure of direct delivery eliminates the delay of stem cells to reach target site of action and hastens the regenerating and rejuvenating process. In the treatment of alopecia, direct delivery has been useful and studied with good results. The other mode of stem cell delivery is systemic delivery (intramuscular, intravenous, intra-articular), where stem cells will be harvested and isolated from its source and will be cultured in laboratory media to exponentially escalate stem cell count to be transplanted [
64]. Regeneration of hair follicles caused by MSCs may be due to reversal of the pathophysiology of alopecia, regeneration of partially destroyed hair follicles or stem cell induced formation of new hair follicles [
65,
66,
67].
2.1.1. Adipose Tissue-Derived Cells
MSCs were traditionally obtained from bone marrow, but with advancements in research, adipose derived stem cells (ADSCs), located in fat tissues including subcutaneous fat tissue, are now easily accessible compared to other sources of MSCs [
68,
69]. Subcutaneous tissue mainly comprises of adipocytes along with other cells like MSCs, fibroblasts, and endothelial cells. The adipose tissue is a warehouse of regenerative molecules, various regenerative products that can be derived out of adipose tissue are nano fat, stromal vascular fraction (SVF), MSCs, adipose derived stem cells-conditioned medium (ADSC-CM), and extracellular vesicles (EV).
Nano fat refers to adipose graft with size less than 400 to 600 microns. They are obtained by mechanical fragmentation and filtration. They are rich in ADSCs with few adipocytes and associated cells whereas SVF mainly comprises of MSCs, endothelial cells, pericytes, immune cells and stromal cells but devoid of any adipocytes. MSCs can also be isolated from the adipose tissue and cultured in pure form. SVF and MSCs are differentiated by their respective specific surface markers. ADSC-CM are rich in growth factors and the commercially available preparation is known as AAPE (advanced adipose derived stem cell protein extract) which is obtained from culturing ADSCs and then the proteins secreted by them are extracted in their lyophilized form [
39,
70].
The stromal vascular fraction is made up of a mix of stem cells from adipose tissue, endothelial precursor cells, mature endothelial cells, lymphocytes, pericytes and pre-adipocytes [
71,
72]. SVF contains roughly 0.01% stem cells. SVF’s diverse biological components boost the permeability of endothelial and epithelial progenitor cells, enhancing the regeneration capacity of diseased and damaged tissues. Among other stem cell isolates, SVF is said to have the highest therapeutic efficacy. Traktuev et al. found that VEGF in SVF aids migration and survival of endothelial progenitor cells [
73,
74].
SVF has the appearance of fibroblasts and the characteristics of MSCs. SVF promotes differentiation of diverse cell lineages due to its MSC-like properties. SVF’s cellular composition contains both HSCs (CD-34 and 45) and MSCs (CD-105 and 146) surface markers. SVF cells express several of the same cell surface markers as bone marrow-derived MSCs, including CD-24, 29, 31, 44, 45, 71, 90, 105/SH2 and SH3. SVF has anti-inflammatory and antiandrogenic properties. In addition, being rich in MSCs, they help in hair restoration [
51]. The key function of MSCs is to maintain homeostasis by facilitating recovery after any insult or injury [
75]. ADSCs have the ability to differentiate into tissues of mesenchymal origin. They are also known to secrete bioactive molecules, such as VEGF, hepatocyte growth factor (HGF), insulin-like growth factor (IGF), and platelet-derived growth factors (PDGF). These growth factors act on the surrounding cells to mediate their functions. They have an important role in neovascularization, which is crucial in the pathophysiology of many types of alopecia. The significance of adipose tissue in alopecia came in to lime light because of its ability to increase vascularity of the scalp when autologous fat was injected to the scarred scalp before hair transplantation. When scalp was pre-treated with adipose tissue injections, it bled more during hair transplantation. The same principle of increased vascularity was extrapolated to treat alopecia [
51,
76,
77]. Many studies have shown considerable results in the treatment of alopecia with adipose derived cells and products [
78,
79].
AD-MSCs express CD34 for roughly 8–12 cellular doublings in culture [
71]. Numerous hypotheses exist about the role of pericytes in the stem cell characteristics of SVF. Pericytes are seen in both MSCs and ADSCs, according to Szoke et al. [
80]. However, Traktuev et al. and Crisen et al. claim CD34+ and CD34− pericytes to be the identities of ADSCs, respectively [
74,
81].
When 1 mL/cm
3 of SVF was injected subcutaneous to scalp, there was significant increase in hair density (31 hair/cm
2). Combination of SVF with fat graft increased the density to 44.1 hair/cm
3 compared to the baseline [
82]. Similarly, intradermal injection of 5 mL SVF in 20 patients showed statistically significant increase in hair density and diameter with improvement in hair pull test at 6 months [
83]. SVF-enriched autologous fat demonstrated 14% increased hair count at 6 months [
84]. ADSC-CM has also shown statistically significant increase in hair density and thickness when 4 mL was used via microneedle roller weekly for 12 weeks [
85] [
86]. Similar results were seen when monthly intradermal ADSC-CM was administered for 6 months [
87]. In a study conducted in Korea, a single injection of autologous SVF to scalp led to statistically significant increase in hair density at 3 and 6 months; improvement in keratin score and hair thickness was also noted [
88]. Fakuoka et al., also documented reduction in hair thinning, increased hair quality and number of hair with ADSC-CM [
39]. Addition of PRP to SVF to get PRS (platelet rich stroma) has also been studied in 10 patients with AGA. A single dose of PRS significantly improved hair density. Interestingly, this study also demonstrated new hair growth in hyperkeratotic plugged non-functioning hair follicles [
89]. It is challenging to isolate and quantify the biological components of SVF, despite its greater translational potential, in regenerative medicine. Several researches have suggested that SVF has a high potential for regenerating tissues [
71].
2.1.2. Hair Follicular Stem Cells
Adult stem cells have the innate ability to regenerate damaged or senescent cells which is mediated through intrinsic mechanisms which in turn will control the expression of genes via transcription. Stem cells achieve homeostasis by responding to their surrounding and also ambience based self-signaling. These stem cell-microenvironment interactions moderate cell growth, differentiation and also the renewal and maintenance of stem cell pool till death of tissue [
90,
91,
92,
93].
HFSCs found in the bulge region of hair follicle include epithelial and melanocytic stem cells. They are mostly dormant but they have the innate ability to migrate, proliferate and differentiate in order to maintain homeostasis [
94] The latent HFSCs of the bulge region spawn ‘primed stem cells’ (also known as early progenitor cells) which later produce progenitor cells in hair matrix, these progenitor cells are fast multiplying. The progenitor cells further differentiate and move towards the surface to form inner root sheath and shaft [
95] With the ongoing differentiation of matrix cells, the stem cells located in the bulge undergo self-renewal in the anagen phase and they will remain in the bulge region to become dormant again [
96,
97] Some dormant HFSCs move out of the bulge to form a new bulge and a new set of primed stem cells to start a fresh cycle. This recurrent activity occurs during anagen, catagen and telogen [
98] Not just hair follicle homeostasis, HFSCs also play a role in the generation of interfollicular epidermis, SG, and aid in wound healing [
99,
100,
101].
In AGA, it is shown that HFSCs are normal in number but there is decreased pool of actively multiplying progenitor cells. This observation clearly conveys that the pathology is not in the number of HFSCs but with the regulator of these stem cells by activating or inhibiting it [
102].
A placebo-controlled study was conducted on 11 patients with AGA Norwood stage 3–5 to quantify the isolated HFSCs microscopically and to know the effect of HFSCs procured by centrifugation of fragmented scalp hair follicle obtained through punch biopsy without any culture. 3728 ± 664.5 cells were present in each scalp suspension. CD44 + MSCs derived from hair follicles amounted for 5% ± 0.7%, while CD200+ hair follicle epithelial stem cells from the bulge accounted for 2.6% ± 0.3%. Mean hair count and hair density (29% ± 5% vs. placebo 1% increase) improvement was observed 23 weeks after the last treatment [
67]. Likewise, another placebo-controlled study was conducted to know the efficacy of autologous PRP (A-PRP) and HF-MSCs obtained by using Rigeneracon device in AGA. Patients treated with A-PRP showed increased mean hair count and hair density (31% ± 2%) 12 weeks after the last injection. HF-MSCs group showed significant increase in mean hair density of 30% ± 5% (after 12 weeks), 29% ± 5% (after 23 weeks) when compared to placebo (<1%) [
103]. Another double blinded study with HF-MSCs reiterated the findings of previous studies which showed increased hair density and count. Interestingly, dynamic hair loss was noted in 6 patients after 26 months [
99]. Increased hair density was seen when micro grafts produced from scalp tissue including HD-AFSCs were employed [
52].
2.1.3. Bone Marrow-Derived Cells
Bone marrow is the conventional source of MSCs. Many animal models have shown progression from telogen to anagen phase after intra dermal injection of bone marrow derived mesenchymal cells (BM-MSCs) and they also induced genes involved in hair regrowth [
104]. Both BM-MSCs and ADSCs are rich in MSCs but their concentration and differentiation abilities are different. BM-MSCs have more of osteogenic potential and ADSCs being primarily angiogenic. BM-MSCs retrieval is relatively invasive and hence it is underutilised for hair restoration [
105]. BM-MSCs and follicular stem cells (FSC) are known for their role in the treatment of alopecia. Occipital hair follicles are innately resistant to AGA and hence are preferred when FSCs are utilized [
56]. The preparation of bone marrow aspirate concentrate (BMAC) follows double centrifugation technique called differential or density centrifugation [
56,
106].
In the normal morphogenesis of skin and its appendages, Wnt and BMP signaling plays a vital role. After culture MSCs detach and form dermal papilla like tissue (DPLT). The DPLT was similar to the human dermal papilla cells (DPC). Growth factors, anti-inflammatory and angiogenic factors also play a role in improving hair growth. The progenitor cells account for 0.001% to 0.01% of BMAC by gradient centrifugation. Growth factors, such as PDGF, TGF-β and BMP-2 and -7 have been shown to have anabolic and anti-inflammatory effects, and it is worth noting that these are present in higher concentrations in BMAC. MSCs induce the synthesis of IL-1Ra and IL-1 molecules in substantial quantities, and these molecules carry out the bioactivity of blocking IL-1 catabolism [
107,
108,
109].
Yoo et al. investigated the use of MSCs from bone marrow and umbilical cord in human hair proliferation in vitro. DPLTs produced by their method had characteristics similar to actual DPC (Size, shape and protein structure), which was demonstrated by microscopy and immunohistochemistry. Transplanted DPLTs also stimulated the growth of new HFs in athymic mice [
109].
There is only one human study published in relation to the utilization of BMAC with good sample size. The purpose of this study was to assess the efficacy of autologous bone marrow mononuclear cells (BMMC) and FSC in AA and AGA. It was carried out on 40 patients, who were divided into four groups of ten each namely patients with AA receiving intradermal BMMC, patients with AA receiving intradermal FSC, patients with androgenetic alopecia receiving intradermal BMMC and patients with AGA receiving intradermal FSC. There was significant improvement 6 months after stem cell therapy, which was evaluated using immunostaining and digital dermoscopy. There was no difference between BMMC and FSC groups and no adverse effects were reported [
56].
There are very few human studies to evaluate the efficacy of BMAC and BMMC, the biology of BMAC has to be well understood for clinical applications. Future of hair restoration lies in the regenerative molecules. Bleeding, infection and persistent pain are the known adverse effects during bone marrow aspiration. BMAC being autologous and easily accessible will play a major role in future.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14030612