Tumor immunotherapy holds great potential for the future of advanced tumor therapy. The application of CD for immunotherapy provides new opportunities for overcoming various obstacles. This research thus makes a comprehensive summary on the application of CD for immunotherapy with an emphasis on the role, function, and reported strategies of CD in mediating immunotherapy.
- cyclodextrin
- cancer
- immunotherapy
1. Introduction
In recent years, significant progress has been made in the field of immunotherapy, and the use of polymeric materials to mitigate the deficiencies of immunotherapy makes them very popular. The special structure of CD has led to its extensive research in different fields.In tumor immunotherapy, CD analogues can be used as immune drugs, or they can be functionalized to form complexes with immune drugs that are originally poorly water-soluble, improving the water solubility of immunotherapeutic drugs to enhance their efficacy. In addition, CDs can also be modified to introduce targeting groups, so that immune drugs can bind specifically to target cells and improve the precision of immunotherapy. In addition, CDs can also carry immune escape or immunosuppressive drugs while encapsulating immune drugs, so that the shortcomings of immunotherapy can be improved and the effect of immunotherapy drugs can be enhanced! The application of cyclodextrins in tumor vaccines also provides a new therapeutic direction for immunotherapy. The number of articles addressing the application of CDs in immunotherapy is not very large. Therefore, CD-mediated immunotherapy is still in the early stages of development. The pursuit of appropriate antitumor immunotherapeutic approaches is the key to addressing cancer challenges, and the integration of tumor immunotherapy with other therapeutic modalities is imperative. Undoubtedly, CD-mediated immunotherapy has emerged as an effective and promising strategy for antitumor immunotherapy. Looking ahead, the utilization of CDs in immunotherapy has great potential for clinical translation in cancer therapy.
2. Application of Cyclodextrins in Cancer Immunotherapy
2.1. Cyclodextrins as Immunomodulators
2.2. Cyclodextrin Host–Guest Recognition, Loading Small Molecule Immunomodulators
Based on the unique structural characteristics of CD, host—guest complexation is the main strategy for the production of complexes based on CD and pharmaceutical preparations. In the context of prostate cancer treatment, Sun’s silicic acid-bound CD/CSF-1R siRNA supermolecule nanoparticles can target the siglec-1 (CD169) overexpressed by M2 macrophagesto obtain the up-regulated CSF-1/CSF-1R signaling pathway, which is related to poor prognosis of cancer patients (Figure 1).
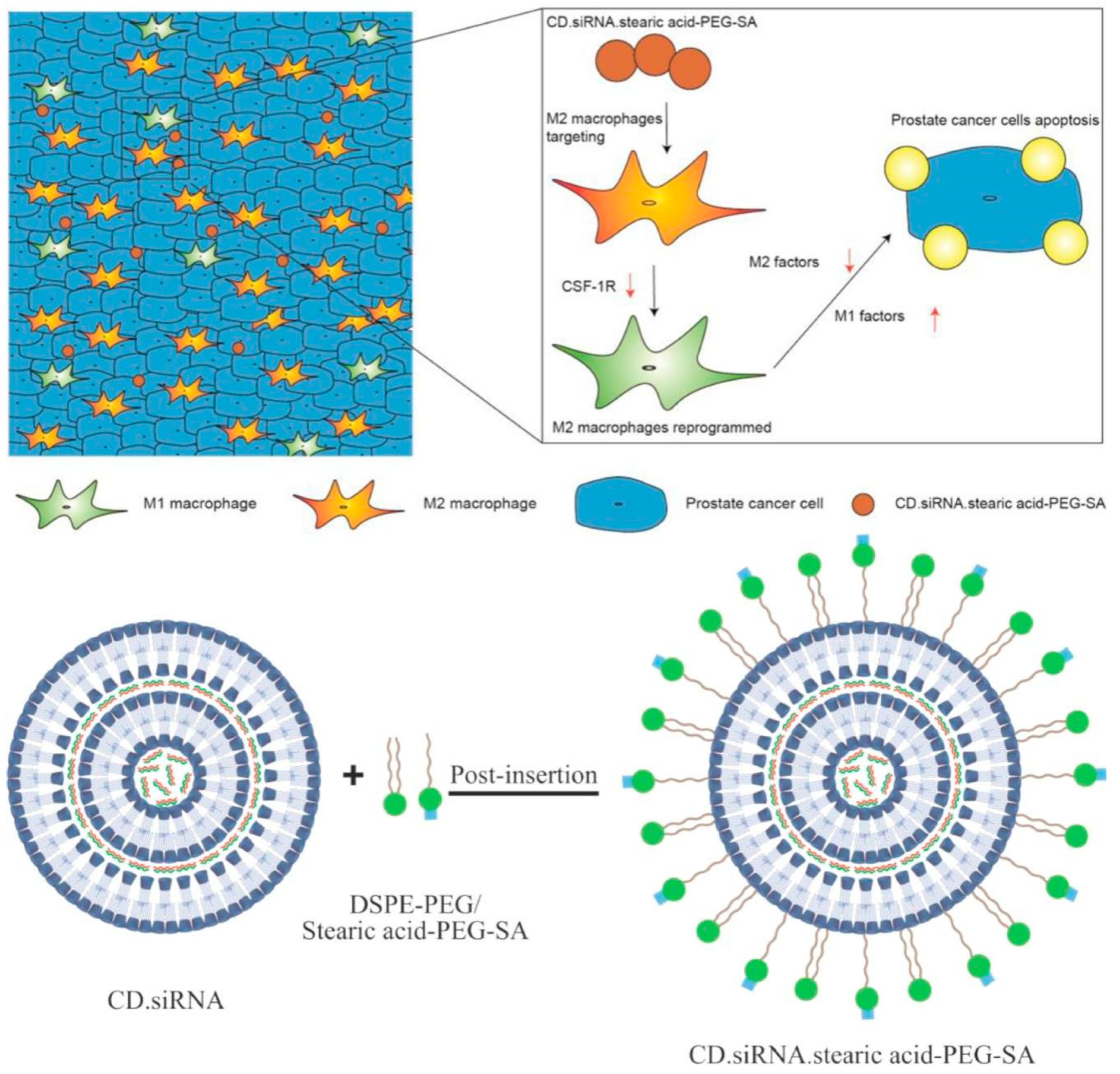
For the application of CD in tumor macrophages, Rodell et al. proposed a TAM re-education strategy. The systematic TAM targeting properties, due to the glycopolymeric nature, good biosafety and degradability of cyclodextrins, have made CDs an excellent candidate. The team loaded the TLR 7/8 agonist with the best re-education TAM effect onto cyclodextrin by host–guest recognition to form nanoparticles and deliver them to the body to exert immunotherapy .
In the field of tumor vaccines, Zhang et al. reported a supramolecular assembly programmable nanodrug (PIAN) as an in situ cancer vaccine. PIAN is fabricated via β-CD/Ad host–guest interactions among PPCD, mPEG TK Ad (PEG thione adamantane) and CpG/polyamide amine thione adamantane (CpG/PAMAM-TK Ad)(Figure 2).
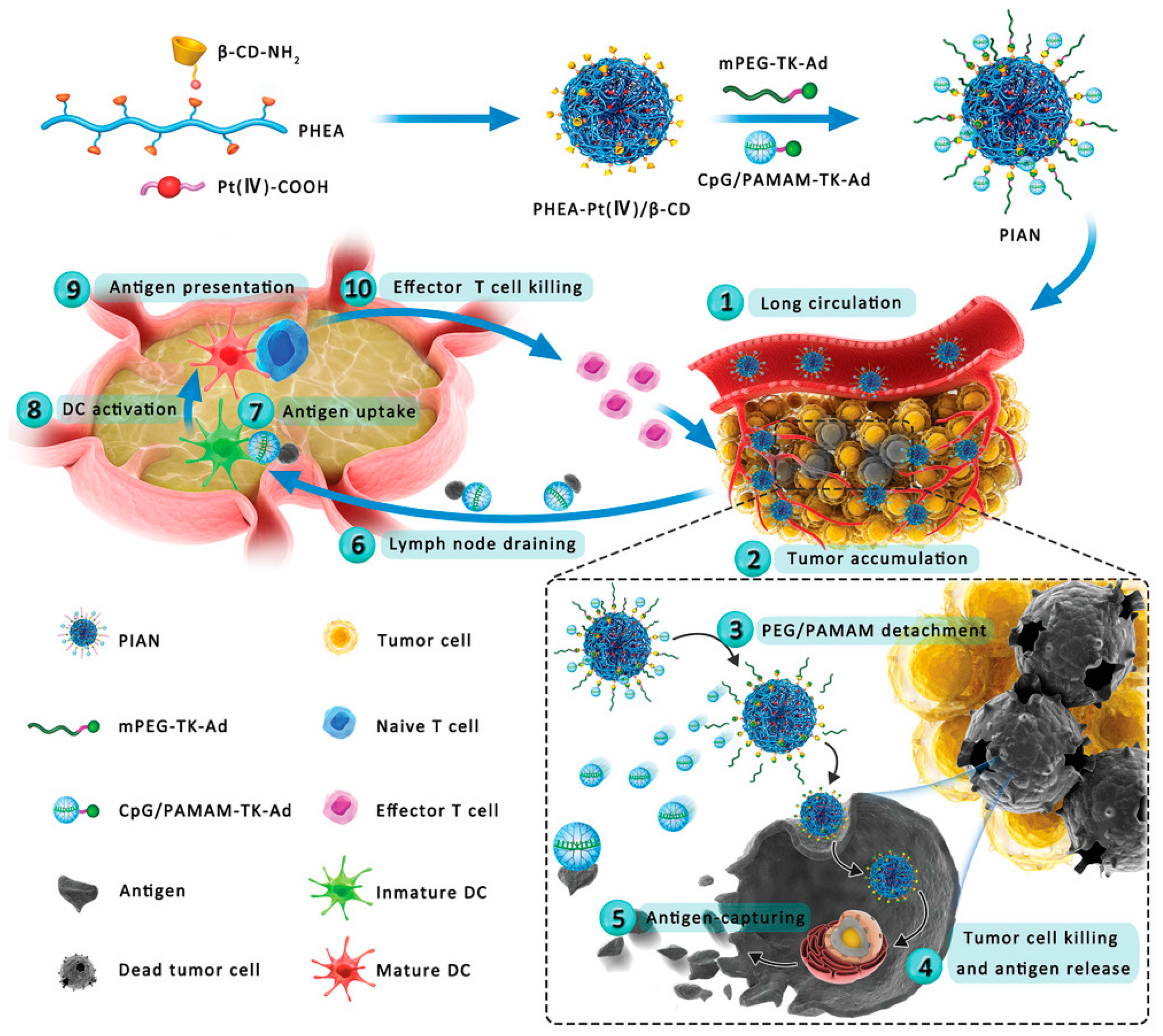
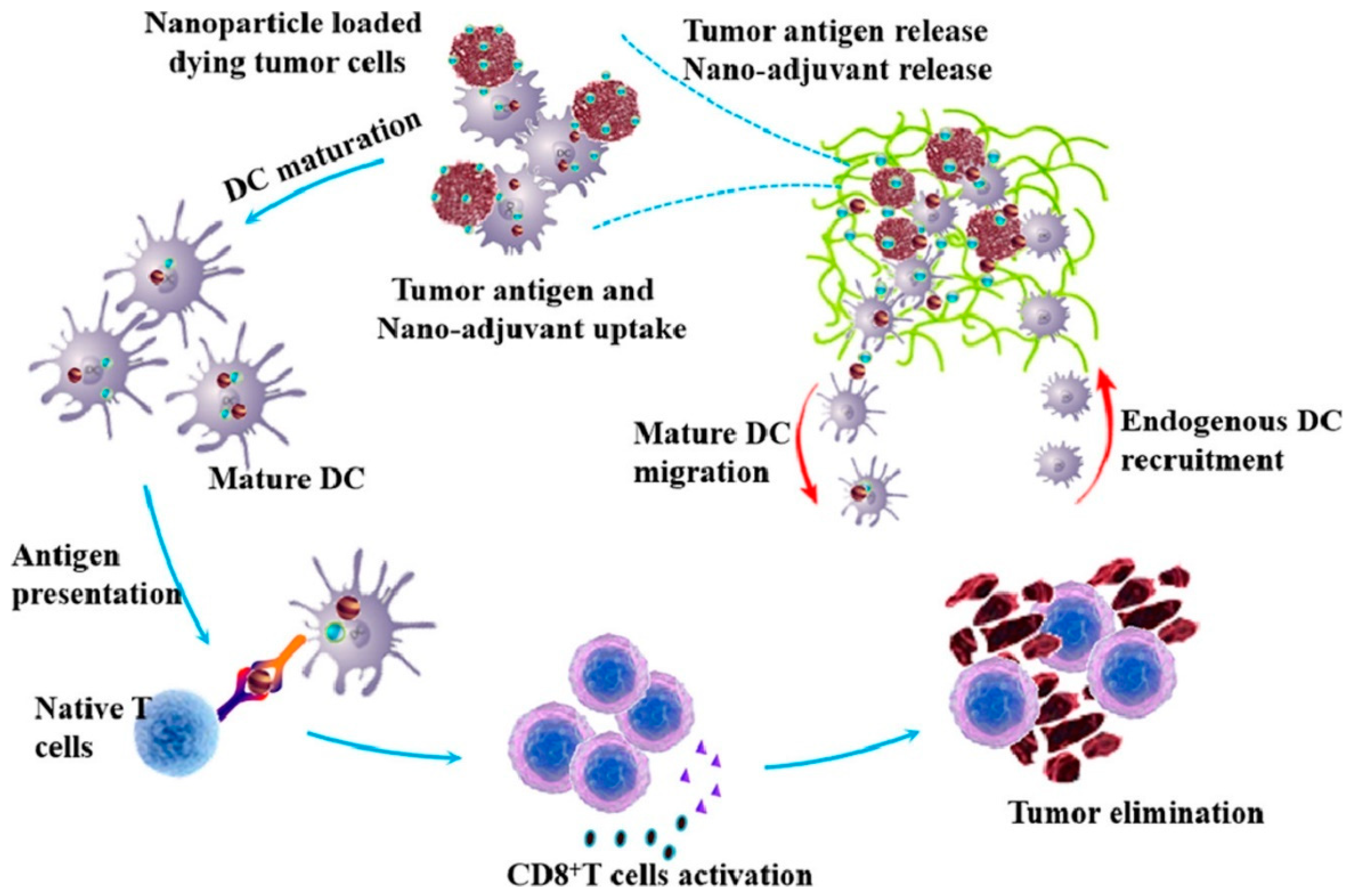
2.3. Cyclodextrin-Bonded Small Molecule Immunomodulators
3.Combination of Immunotherapy and Other Therapies
3.1. Immunotherapy and Chemotherapy
Some chemotherapy drugs can induce immunogenic death of tumor cells, which involves the release of antigens from tumor cells, uptake and presentation by DC cells, and activation of cytotoxic T lymphocytes. Paclitaxel (PTX) is a commonly used broad-spectrum chemotherapy drug with poor water solubility. Some studies have shown that low-dose PTX can regulate the immunosuppressive tumor microenvironment to improve the immunotherapeutic effect of cytokines. In addition, low-dose PTX can also expose calcium reticulin (CRT) on tumor cells, stimulate DC cells, and rebuild immune monitoring. Song et al. constructed a responsive nano gel for the tumor microenvironment. This nanogel enables co-delivery of PTX and IL-2( Figure 5).
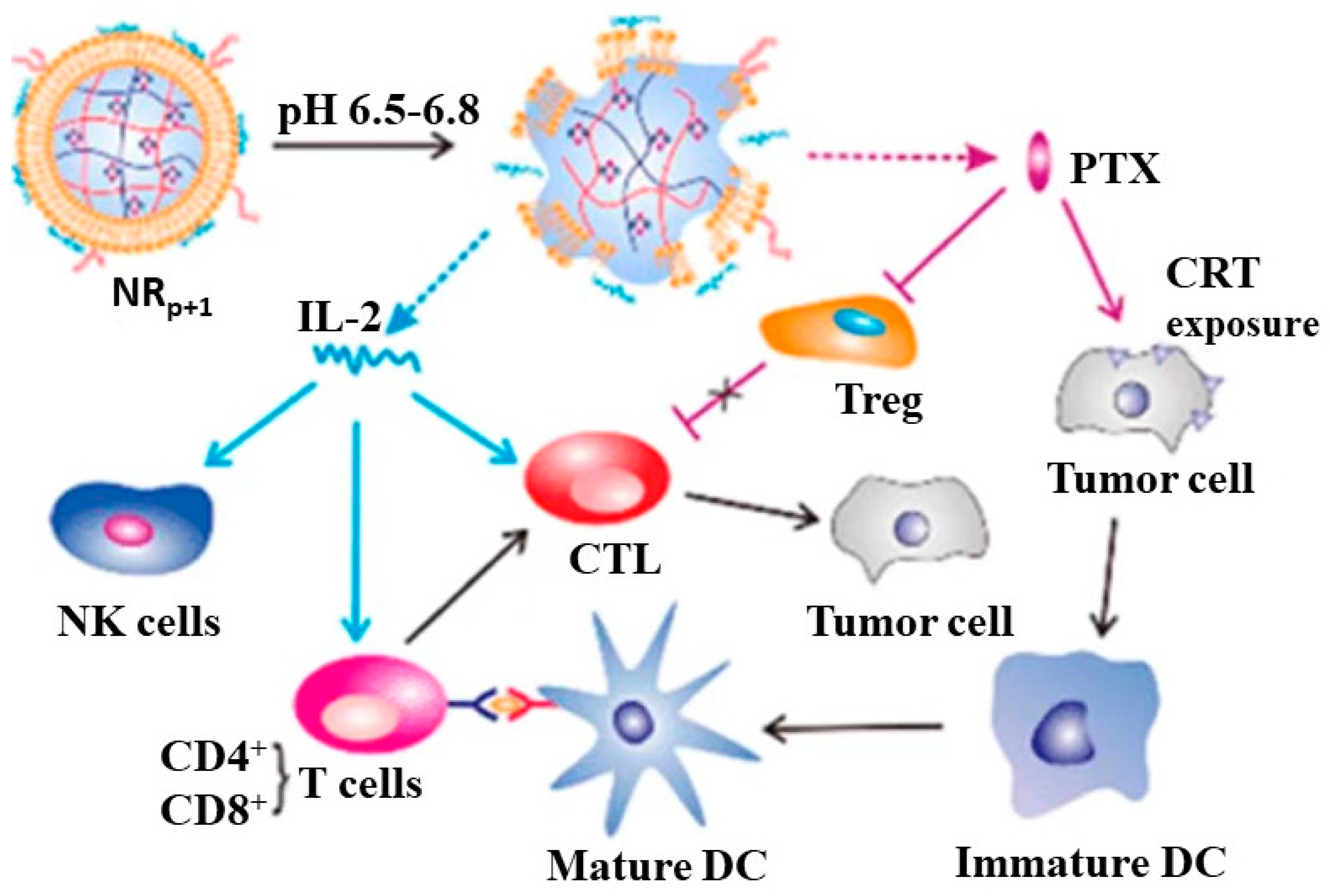
Figure 5. Tumor microenvironment responsive nanogel for the combinatorial antitumor effect of chemotherapy and immunotherapy.
NLG919 has been shown to effectively block IDO-1-mediated immunosuppression]. However, clinical trials have shown that NLG919 is insoluble in water and cannot be administered by injection. Therefore, a drug carrier that can readily load hydrophobic compounds with commendable biocompatibility and minimal toxicity is necessary. CDs and their derivatives manifest exceptional characteristics that fulfill these prerequisites. Xu J et al. conducted a comprehensive evaluation of the interaction between various CDs and NLG919, identifying the optimal carrier for NLG919 administration. Among these, HP-β-CD emerged as the most advantageous for facilitating NLG919 dissolution and subsequent intravenous injection. Furthermore, when combined with paclitaxel (PTX), the cytotoxicity toward tumor cells exhibited a substantial augmentation. Notably, the NLG919/HP-β-CD complex demonstrated heightened chemotherapeutic potency in synergy with PTX, thereby attaining immuno-chemotherapeutic synergy and amplifying the overall antitumor efficacy (Figure 6).
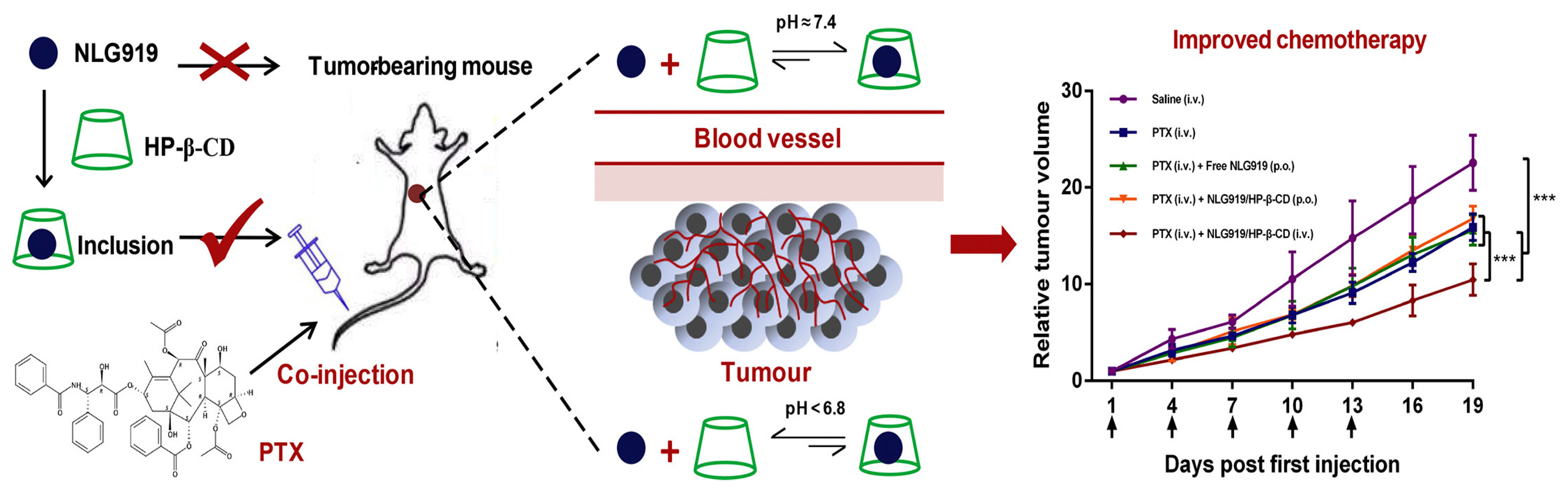
Figure 6. Schematic diagram of NLG919/cyclodextrin complexation combined with paclitaxel, and the relative tumor volume data, *** p < 0.001, n = 6.
An unprecedented “mutual cooperation” between CD and paclitaxel was reported for advanced melanoma therapy. Specifically, a multivalent host–guest interaction composite (pPTX/pCD-pSNO) was constructed by host/guest inclusion complexation between a nitric oxide (pSNO)-modified β-CD polymer and a polymerized paclitaxel (pPTX). Compared with an oncolytic virus (OV) approved for the treatment of advanced melanoma, this nano-assembly has the advantages of better safety and controllability (Figure 7).
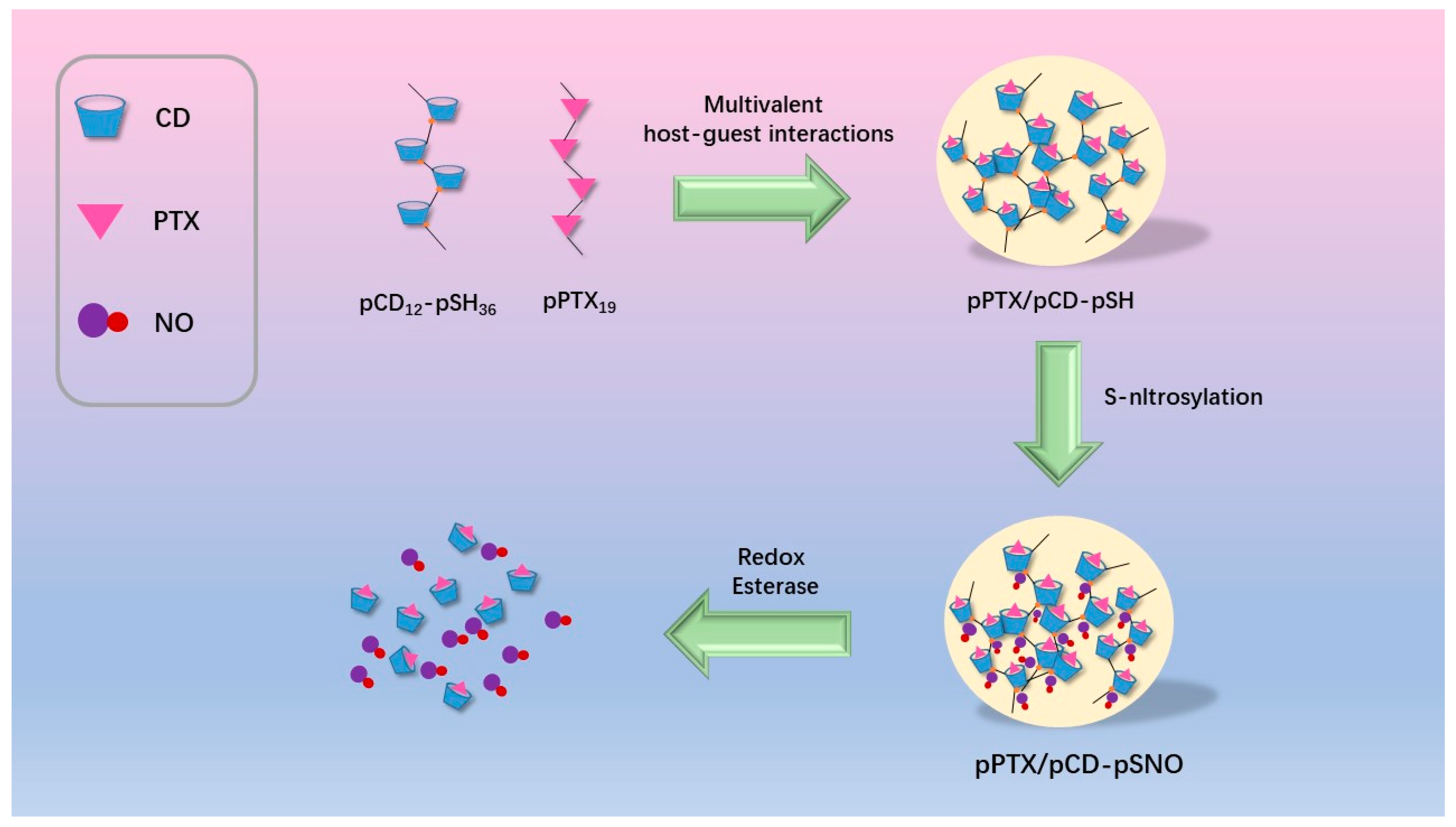
Figure 7. Schematic diagram of Poly(cyclodextrin)-drug nanocomplexes(pPTX/pCD-pSNO).
Safiye Akkın et al. devised a CD nanocomposite through charge interaction, aiming to specifically target the widely used chemotherapy drugs fluorouracil (5-FU) and interleukin 2 (IL-2) in the context of cancer treatment. Extensive experimental studies have consistently demonstrated that the CD nanocomposites possess a drug loading capacity of approximately 40% for 5-FU and an impressive drug loading capacity of 99.8% for IL-2. Animal studies have revealed the compound's commendable safety profile in healthy mice, as well as its potent anticancer activity against colon cancer cells in CT26 mice, outperforming the efficacy of 5-FU as a standalone drug (Figure 8).
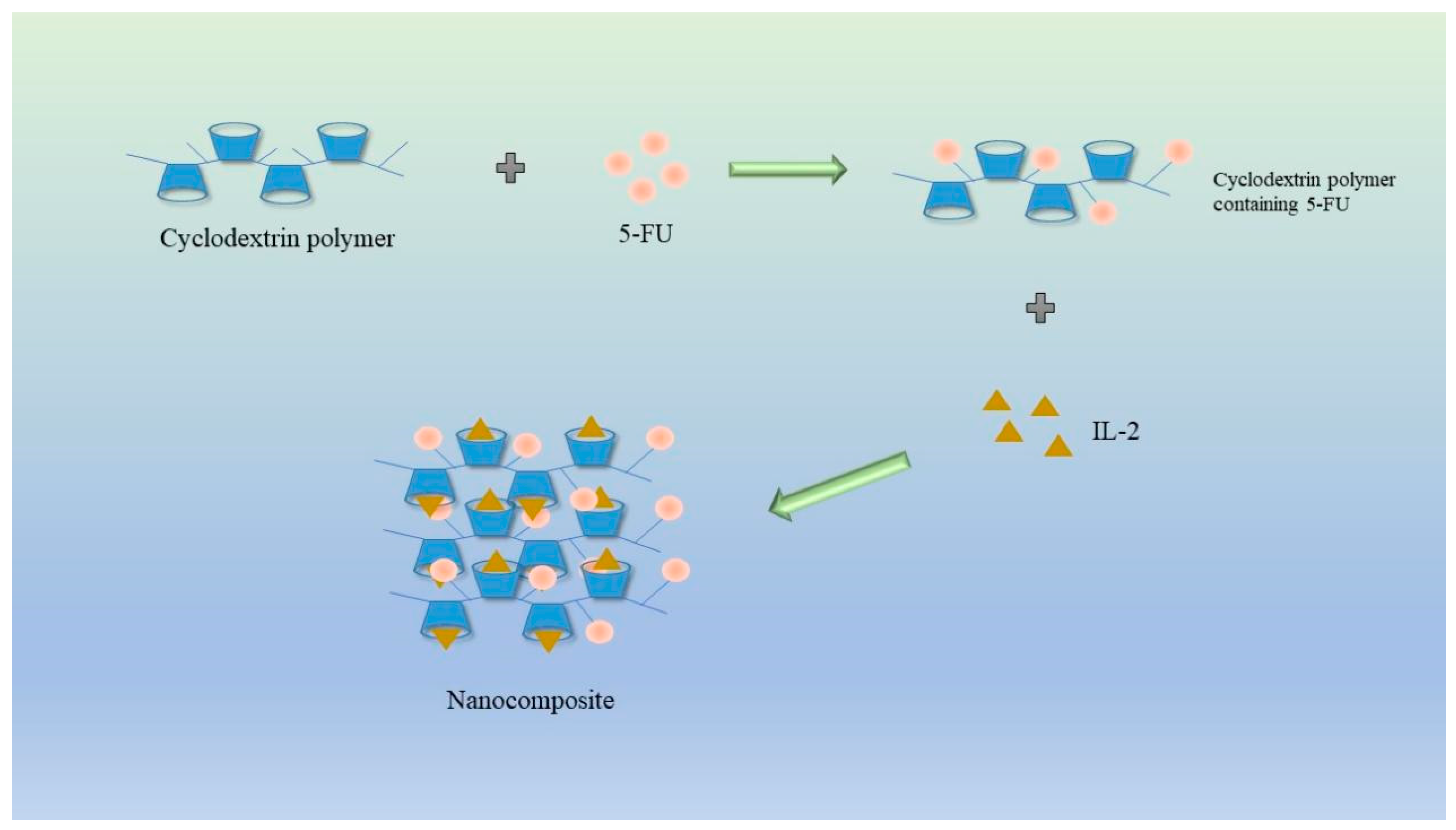
Figure 8. Schematic diagram of cyclodextrin and interleukin-2 nanocomplex loaded with 5-FU for the treatment of colon cancer.
In terms of DOX, Hu et al. reported a GSH responsive supermolecule gold nanocage based on poly cyclodextrin. When the gold nanocage enters the tumor site, due to high GSH, the disulfide bond of the gold nanocage will be destroyed, and PDOPNCS will be dissociated. This gold nanocage prevents DOX from expelling tumor through GSH, enhances chemotherapy activity, and induces cell immune death (ICD), thus generating a synergistic effect of immunochemotherapy (Figure 9).
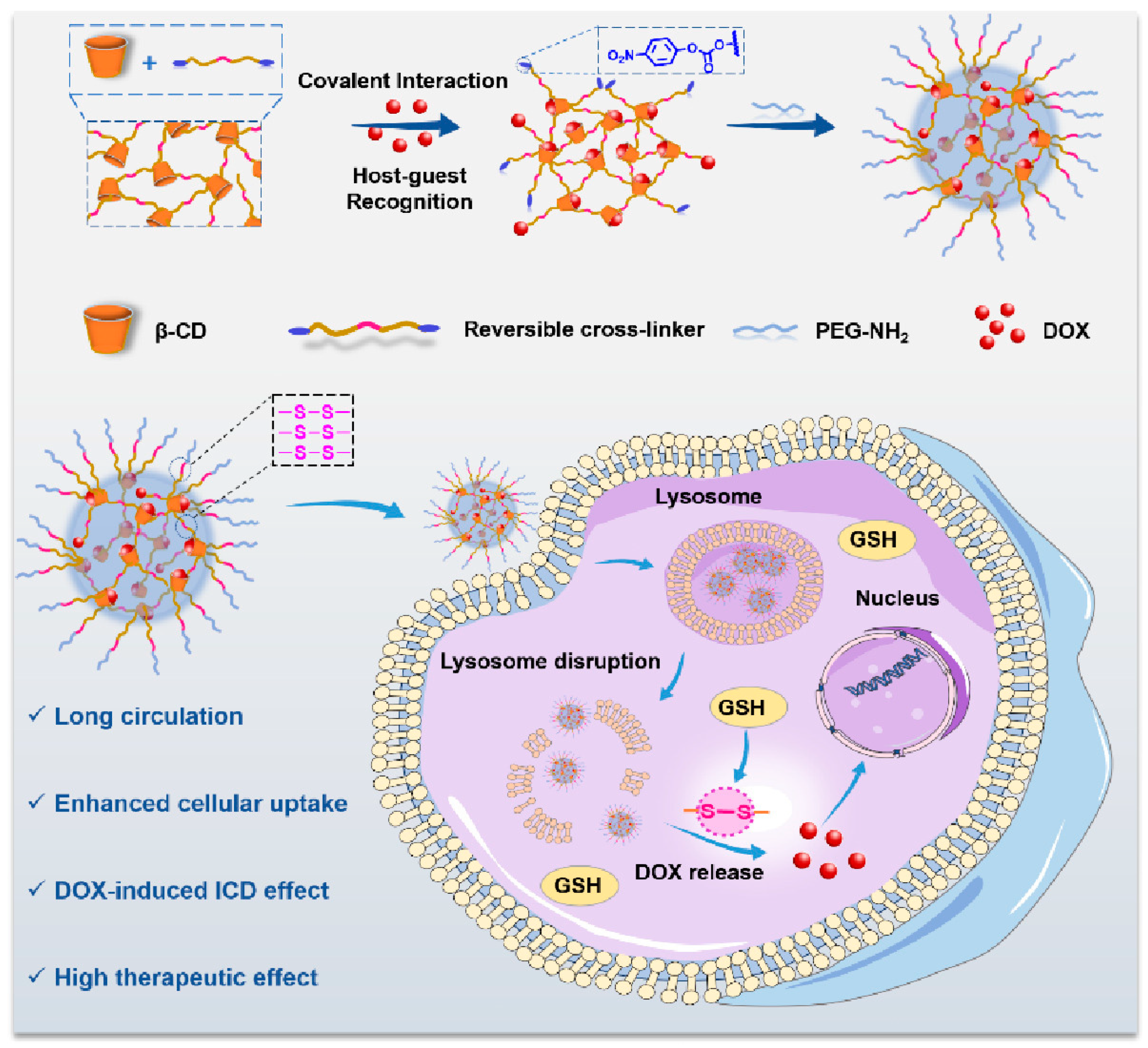
Figure 9. Schematic diagram of reduction triggered polycyclodextrin supermolecule gold nanocage.
3.2. Immunotherapy and Photothermal Therapy
Photothermal therapy (PTT) currently stands as one of the most prominent therapeutic modalities. Noteworthy attributes of this therapy include the absence of systemic toxicity, robust specificity, and remarkable therapeutic efficacy. Photothermal therapy boasts a longstanding history and was predominantly employed in the treatment of skin ailments, including psoriasis and related conditions. The efficacy of photothermal therapy is contingent upon the tissue-penetrating capacity of near-infrared light (NIR). However, the efficacy of photothermal therapy is also constrained by the limitations associated with light penetration. The amalgamation of photothermal therapy with complementary modalities presents a viable avenue for enhancing the therapeutic outcomes of both approaches.
Liu J et al. designed a nanoplatform based on gold nanorods(GNRCDP8MA) for NIR II-mediated photothermal therapy (PTT). GNRCDP8MA specifically targets prostate cancer cells and selectively aggregates at tumor sites in vivo. In addition, under 1208 nm laser irradiation, GNR-CDP8MA almost completely eliminated prostate cancer cell-derived tumors (Figure 10).
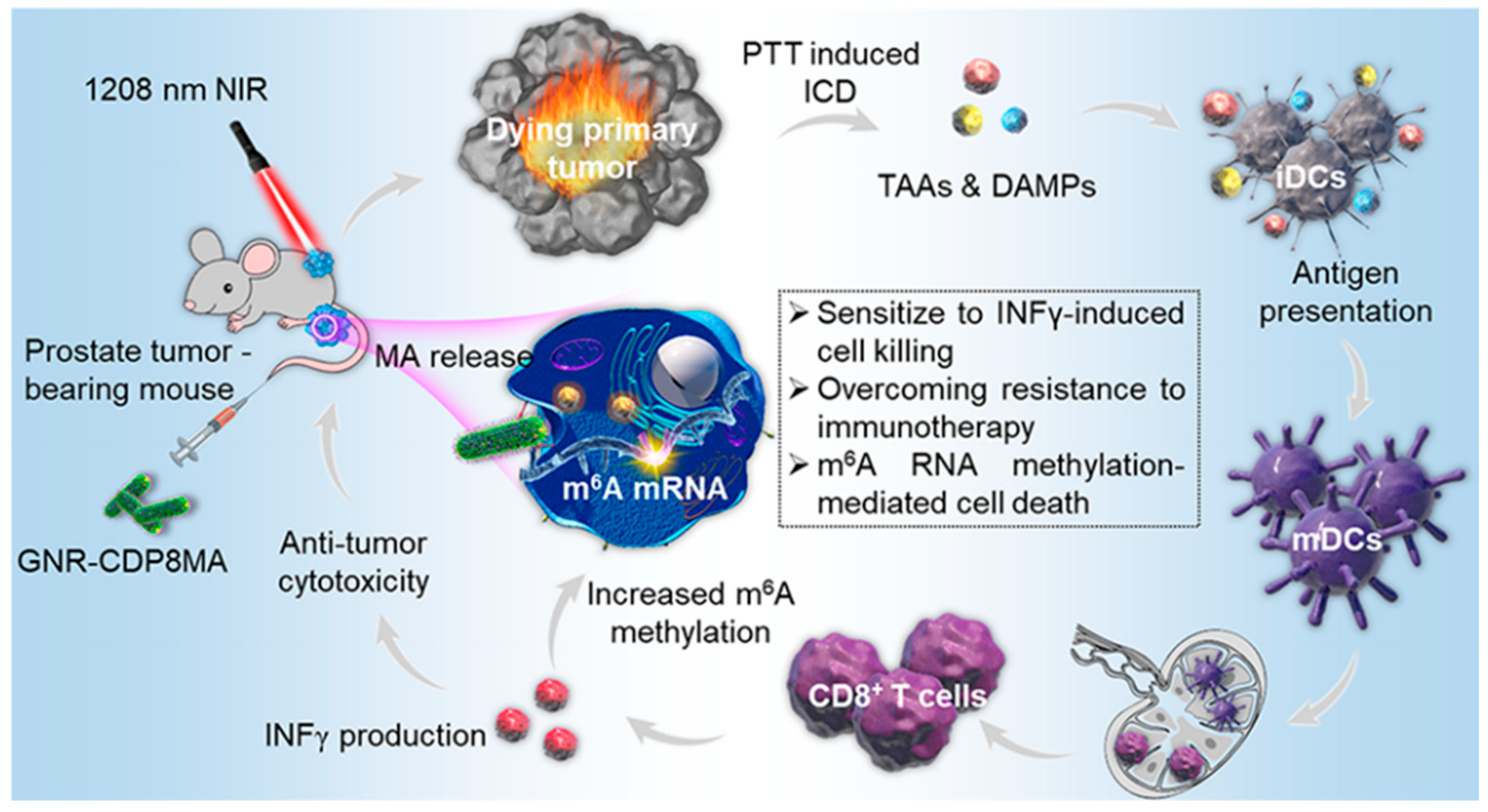
Figure 10. Cyclodextrin-functionalized gold nanorods loaded with meclofenamic acid for improving N6-methyladenosine-mediated→second→near-infrared photothermal immunotherapy.
The integration of CD-mediated immunotherapy and photothermal therapy has also found application in cancer vaccine development. Qin L et al. proposed a novel approach by utilizing the photothermal agent indocyanine green (ICG) to encapsulate doxorubicin (DOX), resulting in the α-CD gel system DOX/ICG/CpG-P-ss-M/CD for combined treatment (Figure 11).
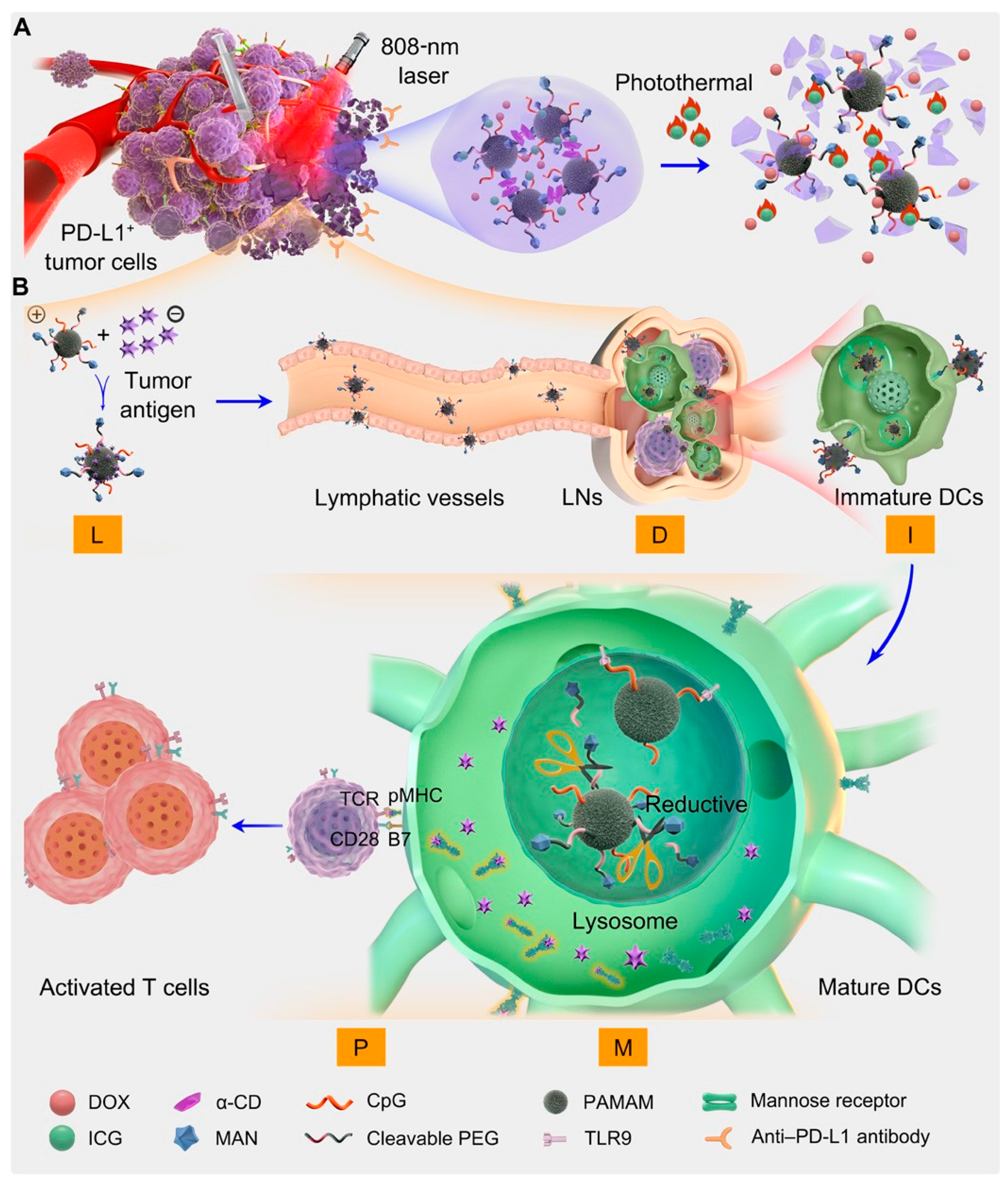
Figure 11. (A) Fabrication of the integrated regimen and the release process of CpG-P-ss-M. (B) Simplified mechanism of CpG-P-ss-M–mediated DC maturation for cancer immunotherapy. Letters LDIMP in orange frame represent loading tumor-specific antigens by DDS, draining to LNs, internalization by DCs, DC maturation for costimulatory molecule expression, and presenting peptide–MHC-I complexes to T cells, respectively.
3.3. Immunotherapy and Photodynamic Therapy
Photodynamic therapy (PDT) has gained significant popularity in recent years as an effective therapeutic approach. Similar to photothermal therapy, PDT also involves the utilization of photosensitizers, but its primary focus lies in the generation of intracellular reactive oxygen species (ROS). However, the efficacy of photodynamic therapy is influenced by the penetration of near-infrared light within tissues and cells. Qi et al. innovatively combined photodynamic therapy with CD immunotherapy to develop an enhanced approach for breast cancer treatment. This nano platform demonstrated passive tumor accumulation and exhibited excellent photodynamic therapy effects under near-infrared light irradiation.
Immunotherapy with PDT and NK cells has shown great potential in addition. However, the activation of NK cells within the tumor microenvironment is inhibited by the shedding of NK group 2, member D ligands (NKG2DLs). Liu et al. constructed a microenvironmental, light-responsive bionanosystem (MLRN) consisting of β-CD containing SB-3CT and liposomes loaded with photosensitizers. SB-3CT promoted both NKG2D/NKG2DL by antagonizing the MMP-2 pathway to block tumor cell immune escape.
The combination of multiple therapeutic approaches has been a hot topic in recent years, and the combination of immunotherapy involving CD with other therapies can improve the therapeutic efficacy through synergistic effects while improving the original shortcomings of each therapy. In the future, the combination of CD-involved immunotherapy with other therapies will lead to new discoveries in oncology treatment.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28145610
