Naringin is a nutritional flavanone glycoside that has been shown to be effective in the treatment of a few chronic disorders associated with ageing. Citrus fruits contain a common flavone glycoside that has specific pharmacological and biological properties. Naringin, a flavone glycoside with a range of intriguing characteristics, is abundant in citrus fruits. Naringin has been shown to have a variety of biological, medicinal, and pharmacological effects. Naringin is hydrolyzed into rhamnose and prunin by the naringinase, which also possesses l-rhamnosidase activity. D-glucosidase subsequently catalyzes the hydrolysis of prunin into glucose and naringenin. Naringin is known for having anti-inflammatory, antioxidant, and tumor-fighting effects. Numerous test animals and cell lines have been used to correlate naringin exposure to asthma, hyperlipidemia, diabetes, cancer, hyperthyroidism, and osteoporosis.
- naringin
- flavonoid
- extraction
- bioactive potential
- pharmaceutical
1. Introduction
2. Chemical Composition of Naringin
2.1. Significance of Flavonoids
| Flavonoids | Examples | Chemical Structure with Molar Mass (g/mol) | Food Sources | Reference |
|---|---|---|---|---|
| Anthocyanin | Cyanidin, pelargonidin, peonidin |
 Cyanidin (287.24) |
Solanum melongena, Rubus fruticosus, Ribes nigrum, Vaccinium sect. Cyanococcus |
[4][5] |
| Flavan-3-ol | Catechin, epicatechin, epigallocatechin |  Catechin (290.26) |
Green tea, Chocolate, Phaseolus vulgaris L., Prunus avium | [5] |
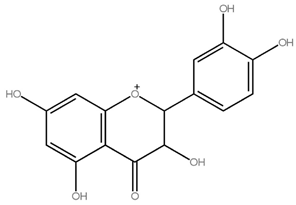
| Flavanones | Hesperidin, Naringin, Eriodictyol |
 Naringin (580.54) |
Orange juice, grapefruit juice, lemon juice | |
| Flavanones | Apigenin, luteolin |  Apigenin (270.05) |
Petroselinum crispum, Apium graveolens, Capsicum annuum | [6] |
| Flavonols | Quercetin, kaempferol, myricetin |
 Quercetin (302.23) |
Allium cepa, Malus domestica, Brassica oleracea var. sabellica, Allium porrum | [7] |
| Isoflavones | Genistein, daidzein, glycitein |  Genistein (270.24) |
Soyflour, soymilk, Glycine max. | [8][9] |
The two primary groups of phenolic chemicals present in citrus fruits are flavonoids and phenolic acids. Citrus flavonoids have been proven to have anti-cancer, anti-inflammatory, anti-aging, anti-bacterial, hepatoprotective, and cardiovascular protective effects, according to several studies. The primary class of phytochemicals found in citrus fruits, particularly in the pulp, peels, and seeds, are called flavonoids. Flavones, flavanones, and flavonols are the three main categories of citrus flavonoids. Table 2 depicts the classification of citrus flavonoids isolated in citrus species, major fruit sources, C-ring structures, and substitution patterns [10].
| Flavonoid | Molecular Weight | C-Ring Structure | Fruit Sources | Substitution Pattern | Reference |
|---|---|---|---|---|---|
| Naringin | 580.541 g/mol | FLA FLA |
Citrus paradisi Citrus aurantium |
5,4′-OH 7-O-Neo |
[11][12] |
| Neoeriocitrin | 596.5 g/mol | FLA | Citrus aurantium | 5,3′,4′-OH 7-O-Neo |
[7][11] |
| Diosmin | 608.54 g/mol | FLO | Citrus sinensis Citrus limonia |
5,3′-OH 4′-OMe 7-O-Rut |
[10] |
| Hesperidin | 610.1898 g/mol | FLA | Citrus sinensis | 5,3′-OH, 4′-OMe 7-O-Rut |
[13] |
| Rutin | 610.517 g/mol | FOL | Citrus limonia | 5,7,3′,4′-OH 3-O-Rut |
[13][14] |
| Naringenin | 272.257 g/mol | FLA | Citrus paradisi | 5,7,4′-OH | [15][16] |
| Hesperetin | 302.27 g/mol | FLA | Citrus sinensis | 5,7,3′-OH 4′-OMe |
[12][17] |
| Kaempferol | 286.23 g/mol | FOL | Citrus paradisi | 5,7,3,4′-OH | [11] |
| Quercetin | 302.236 g/mol | FOL | Citrus limonia | 5,7,3,3′,4′-OH | [13] |
| Tangeretin | 372.37 g/mol | FLO | Citrus aurantium Citrus paradisi Citrus limonia |
5,6,7,8,4′-OMe | [18] |
| Luteolin | 286.24 g/mol | FLO | Citrus limonia Citrus aurantium |
5,7,3′,4′-OH | [19] |
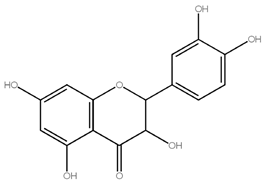
2.2. Structure of Naringin
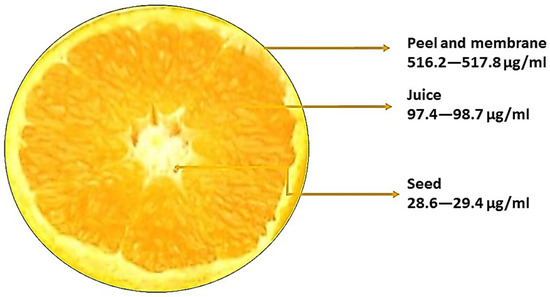
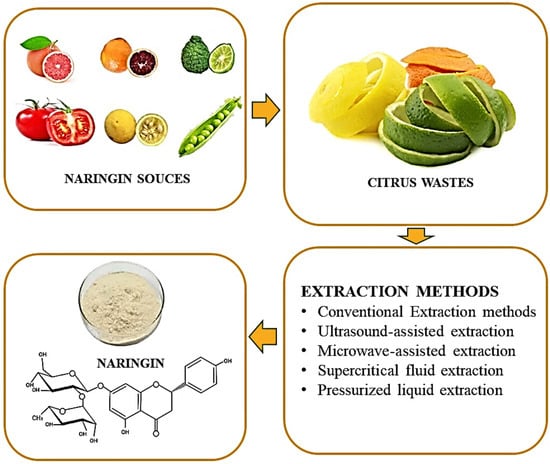
3. Sources of Naringin
4. Extraction of Naringin
5. Possible Health Benefit
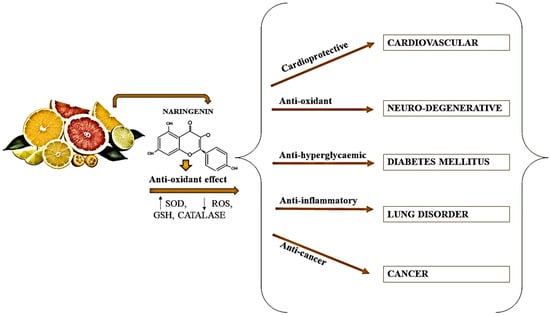
5.1. Anticancer Properties of Naringin
5.2. Antidiabetic Properties of Naringin
5.3. Anti-Inflammatory Properties of Naringin
5.4. Hepatoprotective Properties of Naringin
5.5. Pharmacokinetics of Naringin
6. Application of Naringin
6.1. In Cosmetic Industry
6.2. Pharmaceutical Application
6.3. In Livestock Sector
6.4. Food Industry
This entry is adapted from the peer-reviewed paper 10.3390/molecules28155623
References
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417.
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a potential anti-obesity target: A review of pharmacological treatment and natural products. Diabetes Metab. Syndr. Obes. 2021, 14, 67–83.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Nawaz, R.; Abbasi, N.A.; Khan, M.R.; Ali, I.; Hasan, S.Z.U.; Hayat, A. Color development in “Feutrell’s early” (Citrus reticulata Blanco) affects peel composition and juice biochemical properties. Int. J. Fruit Sci. 2020, 20, 871–890.
- Tan, J.; Li, Y.; Hou, D. The Effects and Mechanisms of cyanidin-3-glucoside and Its phenolic Metabolites in Maintaining intestinal Integrity. Antioxidants 2019, 8, 479.
- Puri, M. Updates on naringinase: Structural and biotechnological aspects. Appl. Microbiol. Biotechnol. 2012, 93, 49–60.
- Hollman, P.C.H.; Arts, I.C.W. Flavonols, flavones and flavanols—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1081–1093.
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076.
- Da Silva, F.L.; Escribano-Bailón, M.T.; Pérez Alonso, J.J.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanin pigments in strawberry. LWT—Food Sci. Technol. 2007, 40, 374–382.
- Sharma, K.; Mahato, N.; Lee, Y.R. Extraction, characterization and biological activity of citrus flavonoids. Rev. Chem. Eng. 2019, 35, 265–284.
- Li, Q.; Zhang, N.; Sun, X.; Zhan, H.; Tian, J.; Fei, X.; Liu, X.; Chen, G.; Wang, Y. Controllable biotransformation of naringin to prunin by naringinase immobilized on functionalized silica. J. Chem. Technol. Biotechnol. 2021, 96, 1218–1227.
- Verma, A.K.; Pratap, R. Chemistry of biologically important flavones. Tetrahedron 2012, 68, 8523–8538.
- Billowria, K.; Ali, R.; Rangra, N.K.; Kumar, R.; Chawla, P.A. Bioactive Flavonoids: A Comprehensive Review on Pharmacokinetics and Analytical Aspects. Crit. Rev. Anal. Chem. 2022, 1–15.
- Tang, D.M.; Zhu, C.F.; Zhong, S.A.; Da Zhou, M. Extraction of naringin from pomelo peels as dihydrochalcone’s precursor. J. Sep. Sci. 2011, 34, 113–117.
- Zhang, L.; Song, L.; Zhang, P.; Liu, T.; Zhou, L.; Yang, G.; Lin, R.; Zhang, J. Solubilities of naringin and naringenin in Different Solvents and Dissociation Constants of naringenin. J. Chem. Eng. Data 2015, 60, 932–940.
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104.
- Su, W.; Wang, Y.; Li, P.; Wu, H.; Zeng, X.; Shi, R.; Zheng, Y.; Li, P.; Peng, W. The potential application of the traditional Chinese herb Exocarpium Citri grandis in the prevention and treatment of COVID-19. Tradit. Med. Res. 2020, 5, 160–166.
- Ahmed, O.M.; Mahmoud, A.M.; Abdel-Moneim, A.; Ashour, M.B. Antidiabetic effects of hesperidin and naringin in type 2 diabetic rats. Diabetol. Croat. 2012, 41, 53–67.
- Sharma, A.; Bhardwaj, P.; Arya, S.K. Naringin: A potential natural product in the field of biomedical applications. Carbohydr. Polym. 2021, 2, 100068.
- Aherne, S.A.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutr. J. 2002, 18, 75–81.
- Ioannou, I.; Nouha, M.; Chaaban, H.; Boudhrioua, N.M.; Ghoul, M. Effect of the process, temperature, light and oxygen on naringin extraction and the evolution of its antioxidant activity. Int. J. Food Sci. Technol. 2020, 53, 2754–2760.
- Wang, S.; Yang, C.; Tu, H.; Zhou, J.; Liu, X.; Cheng, Y.; Luo, J.; Deng, X.; Zhang, H.; Xu, J. Characterization and metabolic diversity of flavonoids in Citrus species. Sci. Rep. 2017, 7, 10549.
- Hoffmann, E. Naringin (Hesperidin de Vry). Arch. Pharm. 1879, 214, 139–145.
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, global distribution, and nutritional importance of citrus fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545.
- Pichaiyongvongdee, S.; Haruenkit, R. Comparative studies of limonin and naringin distribution in different parts of pummelo (Citrus grandis (L.) Osbeck) cultivars grown in Thailand. J. Nat. Sci. 2009, 43, 28–36.
- Sir, K.A.; Randa, E.; Amro, A.B. Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219.
- Hassan, R.A.; Hozayen, W.G.; Abo Sree, H.T.; Al-Muzafar, H.M.; Amin, K.A.; Ahmed, O.M. Naringin and hesperidin counteract diclofenac-induced hepatotoxicity in male Wistar rats via their antioxidant, anti-inflammatory, and antiapoptotic activities. Oxidative Med. Cell. Longev. 2021, 2021, 9990091.
- Bahorun, T.; Ramful-baboolall, D.; Neergheen-bhujun, V.; Aruoma, O.I.A.; Verma, S.; Tarnus, E.; Robert, C.; Silva, D.; Rondeau, P. Phytophenolic Nutrients in Citrus: Biochemical and Molecular Evidence. In Advances in Citrus Nutrition; Springer: Berlin/Heidelberg, Germany, 2012.
- Zhao, B.T.; Kim, E.J.; Son, K.H.; Son, J.K.; Min, B.S.; Woo, M.H. Quality evaluation and pattern recognition analyses of marker compounds from five medicinal drugs of Rutaceae family by HPLC/PDA. Arch. Pharm. Res. 2015, 38, 1512–1520.
- Grassino, A.N.; Pedisić, S.; Dragović-Uzelac, V.; Karlović, S.; Ježek, D.; Bosiljkov, T. Insight into high-hydrostatic pressure extraction of polyphenols from tomato peel waste. Plant Foods Hum. Nutr. 2020, 75, 427–433.
- Victor, M.M.; David, J.M.; Sakukuma, M.C.K.; França, E.L.; Nunes, A.V.J. A simple and efficient process for the extraction of naringin from grapefruit peel waste. Green Process. Synth. 2018, 7, 524–529.
- Chávez-González, M.L.; Sepúlveda, L.; Verma, D.K.; Luna-García, H.A.; Rodríguez-Durán, L.V.; Ilina, A.; Aguilar, C.N. Conventional and emerging extraction processes of flavonoids. Processes 2020, 8, 434.
- Ciğeroğlu, Z.; Bayramoğlu, M.; Kırbaşlar, Ş.İ.; Şahin, S. Comparison of microwave-assisted techniques for the extraction of antioxidants from Citrus paradisi Macf. biowastes. J. Food Sci. Technol. 2021, 58, 1190–1198.
- Cheigh, C.I.; Chung, E.Y.; Chung, M.S. Enhanced extraction of flavanones hesperidin and narirutin from Citrus unshiu peel using subcritical water. J. Food Eng. 2012, 110, 472–477.
- Bacanli, M.; Başaran, A.A.; Başaran, N. The major flavonoid of grapefruit: Naringin. In Polyphenols: Prevention and Treatment of Human Disease; Academic Press: Cambridge, MA, USA, 2018; pp. 37–44.
- El Kantar, S.; Rajha, H.N.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Green extraction of polyphenols from grapefruit peels using high voltage electrical discharges, deep eutectic solvents and aqueous glycerol. Food Chem. 2019, 295, 165–171.
- Ma, G.; Zhang, L.; Sugiura, M.; Kato, M. Citrus and Health. The Genus Citrus; Elsevier: Amsterdam, The Netherlands, 2020.
- Yan, Y.; Zhou, H.; Wu, C.; Feng, X.; Han, C.; Chen, H.; Liu, Y.; Li, Y. Ultrasound-assisted aqueous two-phase extraction of synephrine, naringin, and neohesperidin from Citrus aurantium L. fruitlets. Prep. Biochem. Biotechnol. 2021, 51, 780–791.
- Gupta, A.K.; Dhua, S.; Sahu, P.P.; Abate, G.; Mishra, P.; Mastinu, A. Variation in phytochemical, antioxidant and volatile composition of pomelo fruit (Citrus grandis (L.) osbeck) during seasonal growth and development. Plants 2021, 10, 1941.
- Ghanbari-Movahed, M.; Jackson, G.; Farzaei, M.H.; Bishayee, A. A systematic review of the preventive and therapeutic effects of naringin against human malignancies. Front. Pharmacol. 2021, 12, 639840.
- Li, H.; Yang, B.; Huang, J.; Xiang, T.; Yin, X.; Wan, J.; Luo, F.; Zhang, L.; Li, H.; Ren, G. Naringin inhibits growth potential of human triple-negative breast cancer cells by targeting β-catenin signaling pathway. Toxicol. Lett. 2013, 220, 219–228.
- Aroui, S.; Fetoui, H.; Kenani, A. Natural dietary compound naringin inhibits glioblastoma cancer neoangiogenesis. BMC Pharmacol. Toxicol. 2020, 21, 46.
- Ahmed, S.; Khan, H.; Aschner, M.; Hasan, M.M.; Hassan, S.T.S. Therapeutic potential of naringin in neurological disorders. Food Chem. Toxicol. 2019, 132, 110646.
- Mahmoud, A.M. Anti-diabetic effect of naringin: Insights into the molecular mechanism. Int. J. Obes. 2016, 8, 324–328.
- Pandey, V.K.; Shams, R.; Singh, R.; Dar, A.H.; Pandiselvam, R.; Rusu, A.V.; Trif, M. A comprehensive review on clove (Caryophyllus aromaticus L.) essential oil and its significance in the formulation of edible coatings for potential food applications. Front. Nutr. 2022, 9, 987674.
- Chen, F.; Zhang, N.; Ma, X.; Huang, T.; Shao, Y.; Wu, C.; Wang, Q. Naringin alleviates diabetic kidney disease through inhibiting oxidative stress and inflammatory reaction. PLoS ONE. 2015, 10, e0143868.
- Kumar, R.; Clermont, G.; Vodovotz, Y.; Chow, C.C. The dynamics of acute inflammation. J. Theor. Biol. 2004, 230, 145–155.
- Lawrence, T.; Gilroy, D.W. Chronic inflammation: A failure of resolution. Int. J. Exp. Pathol. 2007, 88, 85–94.
- El-Desoky, A.H.; Abdel-Rahman, R.F.; Ahmed, O.K.; El-Beltagi, H.S.; Hattori, M. Anti-inflammatory and antioxidant activities of naringin isolated from Carissa carandas L.: In vitro and in vivo evidence. Phytomedicine 2018, 42, 126–134.
- Pari, L.; Amudha, K. Hepatoprotective role of naringin on nickel-induced toxicity in male Wistar rats. Eur. J. Clin. Pharmacol. 2011, 650, 364–370.
- Chen, J.C.; Li, L.J.; Wen, S.M.; He, Y.C.; Liu, H.X.; Zheng, Q.S. Quantitative analysis and simulation of anti-inflammatory effects from the active components of Paino Powder in rats. Chin. J. Integr. Med. 2011, 201203, online ahead of print.
- Shirani, K.; Yousefsani, B.S.; Shirani, M.; Karimi, G. Protective effects of naringin against drugs and chemical toxins induced hepatotoxicity: A review. Phytother. Res. 2020, 34, 1734–1744.
- Gillessen, A.; Schmidt, H.H.J. Silymarin as supportive treatment in liver diseases: A narrative review. Adv. Ther. 2020, 37, 1279–1301.
- Kawaguchi, K.; Maruyama, H.; Hasunuma, R.; Kumazawa, Y. Suppression of inflammatory responses after onset of collagen-induced arthritis in mice by oral administration of the Citrus flavanone naringin. Immunopharmacol. Immunotoxicol. 2011, 33, 723–729.
- Yang, X.; Zhao, Y.; Gu, Q.; Chen, W.; Guo, X. Effects of naringin on postharvest storage quality of bean sprouts. Foods 2022, 11, 2294.
- Bailey, D.G.; Dresser, G.K. Interactions between grapefruit juice and cardiovascular drugs. Am. J. Cardiol. 2004, 4, 281–297.
- Tsai, Y.J.; Tsai, T.H. Mesenteric lymphatic absorption and the pharmacokinetics of naringin and naringenin in the rat. J. Agric. Food Chem. 2012, 60, 12435–12442.
- Tesseromatis, C.; Alevizou, A. The role of the protein-binding on the mode of drug action as well the interactions with other drugs. Eur. J. Drug Metab. Pharmacokinet. 2008, 33, 225–230.
- Goyal, A.; Verma, A.; Dubey, N.; Raghav, J.; Agrawal, A. Naringenin: A prospec-tive therapeutic agent for Alzheimer’s and Parkinson’s disease. J. Food Biochem. 2022, 46, e14415.
- Turfus, S.C.; Delgoda, R.; Picking, D.; Gurley, B.J. Pharmacokinetics. In Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 2017.
- Pandey, V.K.; Islam, R.U.; Shams, R.; Dar, A.H. A comprehensive review on the application of essential oils as bioactive compounds in nano-emulsion based edible coatings of fruits and vegetables. Appl. Food Res. 2022, 2, 100042.
- Oliva, J.; French, B.A.; Li, J.; Bardag-Gorce, F.; Fu, P.; French, S.W. Sirt1 is involved in energy metabolism: The role of chronic ethanol feeding and resveratrol. Exp. Mol. Pathol. 2008, 85, 155–159.
- Goodarzi-Boroojeni, F.; Männer, K.; Zentek, J. The impacts of Macleaya cordata extract and naringin inclusion in post-weaning piglet diets on performance, nutrient digestibility and intestinal histomorphology. Arch. Anim. Nutr. 2018, 72, 178–189.
- Kandhare, A.D.; Alam, J.; Patil, M.V.K.; Sinha, A.; Bodhankar, S.L. Wound healing potential of naringin ointment formulation via regulating the expression of inflammatory, apoptotic and growth mediators in experimental rats. Pharm. Biol. 2016, 54, 419–432.
- Wang, H.; Hu, H.; Zhang, X.; Zheng, L.; Ruan, J.; Cao, J.; Zhang, X. Preparation, physicochemical characterization, and antioxidant activity of naringin–silk fibroin–alginate microspheres and application in yogurt. Foods 2022, 11, 2147.
