Like many neurodegenerative diseases, Parkinson’s disease (PD) is characterized by the formation of proteinaceous aggregates in brain cells. In PD, those proteinaceous aggregates are formed by the α-synuclein (αSyn) and are considered the trademark of this neurodegenerative disease. In addition to PD, αSyn pathological aggregation is also detected in atypical Parkinsonism, including Dementia with Lewy Bodies (DLB), Multiple System Atrophy (MSA), as well as neurodegeneration with brain iron accumulation, some cases of traumatic brain injuries, and variants of Alzheimer’s disease. Collectively, these (and other) disorders are referred to as synucleinopathies, highlighting the relation between disease type and protein misfolding/aggregation. Despite these pathological relationships, however, synucleinopathies cover a wide range of pathologies, present with a multiplicity of symptoms, and arise from dysfunctions in different neuroanatomical regions and cell populations. Strikingly, αSyn deposition occurs in different types of cells, with oligodendrocytes being mainly affected in MSA, while aggregates are found in neurons in PD. If multiple factors contribute to the development of a pathology, especially in the cases of slow-developing neurodegenerative disorders, the common presence of αSyn aggregation, as both a marker and potential driver of disease, is puzzling.
1. The Strain-to-Cell Connection
As described, pathological αSyn strains only affect certain types of cells. In most studies, αSyn deposition in neurons vs. astrocytes, for example, clearly delineates the ability of various strains to induce pathologies. Induced cellular death also differs and rarely correlates with the presence of inclusions. Selective response of cells might arise from various factors, including the cellular content in αSyn, the possibility of uptake, and induced toxicity through loss/gain-of-function.
Regardless of the strain, emerging evidence associates αSyn expression level and αSyn accumulation. Intracellular αSyn concentration affects the cell’s response to PFFs. Courte et al. treated neurons isolated from diverse parts of the brains with PFFs and found that the levels of phospho-αSyn accumulation were significantly different. In this case, the neurons from the hippocampus presented the most inclusions [
69]. This was despite a similar intake with no observable effect on cell death. The amount of aggregation correlated with the endogenous expression level of αSyn when WT mice were used; reduction in αSyn deposition could be observed when neurons from heterozygous SNCA
+/− mice, with lower αSyn expression, were used. It was also observed that isogenic correction of αSyn levels in human induced pluripotent cells-derived dopaminergic neurons from SNCA triplication patients reduced intracellular αSyn aggregation and cell death after exogenous addition of PFFs or PD-/MSA-derived fibrils [
70]. This is also in line with some observations of familial cases of synucleinopathies. SNCA copy number variations (CNVs), duplications [
71], and triplications [
72] have all been linked to familial forms of the disease. A clear dosage effect was reported, with the severity and rapidity of disease progression increasing with the copy number [
73,
74]. It is noteworthy that although CNVs primarily lead to LB pathology, duplications affect the brainstem region more frequently compared to triplication cases that tend to develop dementia, mimicking the differential spread of synucleinopathies. Yet, the substantia nigra that is mainly affected by PD is not the highest αSyn expressing region of the brain, and more strikingly, oligodendrocytes do not present high endogenous levels of αSyn, to the point where it was long thought that they did not express αSyn at all. Therefore, a higher endogenous concentration of αSyn in a cell may facilitate aggregation, but other factors are certainly at play.
Variable susceptibility between different cell types may also be partially explained by how efficiently αSyn seeds enter the target cells. αSyn aggregates have been shown to diffuse through the cellular membrane or be taken up by macropinocytosis [
75,
76] or phagocytosis in the case of microglia [
77]. In addition to these non-specific mechanisms, αSyn is also actively transported by receptor-mediated cellular uptake [
78], which can be cell-type specific [
76,
79]. An important finding by Mao et al. [
80] was the identification of a membrane-receptor, lymphocyte-activation gene 3 (LAG3), with increased affinity for αSyn aggregates that mediates the transmission of the αSyn pathology [
81]. However, LAG3 may only be one of the receptors involved in αSyn internalization [
82]. Previously, heparan sulfate proteoglycans (HSPGs) [
83], and particularly syndecan 3 [
84], have been shown to bind and mediate the uptake of αSyn aggregates. Syndecans have also been suggested to play a role in mediating fibril formation of not only αSyn [
85] but also Tau [
84] and Aβ fibrils [
86]. This internalization route seems important for oligodendrocyte uptake but is dispensable for microglial or astrocyte uptake, offering an example of selectivity. Interestingly, HSPGs mediate the uptake of a variety of misfolded protein aggregates [
83], and specific uptake can be fine-tuned by varying length and sulfation patterns of the receptors [
87]. Binding to and clustering of different membrane proteins by αSyn aggregates has been studied and shown to correlate with seeding propensity [
88]. As immune cells, microglia and, to a lesser extent, astrocytes expose other classes of membrane receptors compared to neurons or oligodendrocytes. Toll-like receptors (TLRs), a class of pattern recognition receptors that probe for molecular patterns associated with pathogens or danger, can bind to or uptake αSyn aggregates. TLR2 [
89] and TLR4 [
90] bind to αSyn aggregates, triggering both their uptake and pro-inflammatory signalling [
91]. Polymorphism creates differences in the exposed binding surfaces that target specific receptors. α3 unit of the Na
+/K
+ ATPase (α3-NKA) has been proposed to play a significant role in mediating the uptake and toxicity of αSyn aggregates [
88]. Importantly, polymorphs have differential binding affinities for α3-NKA and other membrane proteins, providing a possible explanation for strain-specific cell susceptibility [
92]. Besides the receptor- or endocytosis-mediated entry, αSyn fibrils can propagate between cells by other mechanisms, including direct membrane penetration or tunneling nanotubes [
93]. Of particular interest is the presence of synuclein in extracellular vesicles (EVs). EVs are emerging key players in cell-to-cell communication in the brain and as biomarkers in neurodegenerative disorders [
94]. So far, little is known about the link between strains and their presence in EVs; however, the compositions of EVs differentiate PD and atypical parkinsonism [
95]. Further, the internalization and cellular processing of EVs appear to be cell-type dependent [
95], which could partially explain the cellular selectivity of some disorders.
The modulation of interactions between αSyn aggregates and other proteins extends beyond cellular uptake and may contribute to differences in seeding and toxicity. Indeed, through binding to distinct partners, αSyn strains could differentially impact cellular processes, modulating the induced toxicity, as seen with membrane receptors. αSyn has been shown to interact with multiple partners due to its intrinsically disordered nature and aptitude to self-organize [
96]. The structure of the fibril is directly linked to the selectivity of the interactome in that each strain uniquely exposes residues principally at the N- or C-termini, thus modulating the accessibility to protein-protein interactions. Stephens et al. recently determined that the more exposed the N-terminus and the beginning of the NAC region of αSyn are, the more aggregation-prone monomeric αSyn conformations become [
97]. It was found that solvent exposure of the N-terminus occurs upon release of C-terminus interactions when calcium binds, but the level of exposure and αSyn’s aggregation propensity is sequence and post-translational modification dependent [
97]. Landureau et al. [
98] examined the solvent accessibility of the above-mentioned ribbons and fibrils [
64], resulting from the in vitro aggregation of WT αSyn in different salt conditions. They found that the N-terminus of fibrils was exposed to solvent, but the same sequences were inaccessible in ribbons. This feature and differences in the C-terminal region probably contribute to the selective binding and inhibition of the proteasome by ribbon-like aggregates observed by Suzuki et al. [
56].
αSyn aggregation has been found to be disrupting the autophagy pathway [
99]. Physical interactions between αSyn and autophagy-related proteins from the GABARAP [
100] (Gamma-aminobutyric acid receptor-associated protein) and LC3 [
99] (Microtubule-associated protein 1A/1B-light chain 3) families only occurred with aggregated forms of αSyn. Of particular pathological relevance are interactions with molecular chaperones [
101]. Heat-shock proteins (HSP) are charged with the ability to recognize, stabilize, refold, or tag misfolded proteins for degradation, therefore, play a prominent role in neurodegenerative diseases. αSyn aggregates have been found to bind to different members of the molecular chaperone family (including HSP90, HSC70, DNAJB6, CLU, CRYAB, BAG, etc.), impacting cellular uptake and aggregation kinetics. Post-translational modifications rely on the recognition and binding of kinases to their substrates; therefore, the detection of αSyn by FYN [
102], GRK5 [
103], or Abl [
104] per se could substantially vary depending on conformations. A recent finding by Burmann et al. [
105] presents a fascinating interplay between the two processes, showing that the phosphorylation pattern does influence chaperone selection.
We would be remiss to not briefly mention that αSyn is not the only protein to aggregate in PD, MSA, or DLB. Tau (Tubulin-associated unit) is a highly soluble protein and, like αSyn, is intrinsically disordered [
106]. The microtubule-associated protein contributes to the stability of axonal microtubules in the brain and, in this role, is involved in the regulation of axonal outgrowth and transport [
106]. The binding of Tau to microtubules is regulated by post-translational modification mainly via phosphorylation [
106] and has been detected in some synucleinopathies. Both αSyn and Tau proteins are found in LBs, and the presence of neurofibrillary tangles is frequently noted [
107]. The two disordered proteins have been found to co-aggregate [
108], and αSyn fibrils can seed Tau aggregation [
109]. Guo et al. [
110] showed that repeated seeded fibrillation in vitro led to the creation of a new strain of αSyn, with an enhanced ability to induce αSyn and Tau aggregation in neurons, compared to the initial fibrils. Mixed αSyn/Tau fibrils have increased seeding activity in cells and in vivo [
111]. This study further suggests that conformational strains of αSyn could partially explain the heterogeneity observed in the spectrum of synucleinopathies. Although Tau is not the main constituent protein in PD, MSA, or DLB as established, the emerging data on the presence of structurally different aggregates of Tau [
35,
110] is worth noting as it reiterates the importance in considering all aspects of synucleinopathy strainotyping.
2. Cell-to-Strain Imprinting
As described above, multiple factors can explain why specific strains target different cell types. Moving forward, we will explore how the reciprocal relationship in which the cellular environment can, in turn, construct conformational strains.
Intuitively, one would expect the cell environment to have a significant impact on the ab nihilo aggregation of αSyn. Peng et al. [
46] showed that incubating monomeric αSyn in either oligodendrocytes or neuron lysates led to the creation of strains with different properties. In particular, oligodendrocytic strains led to increased development of a phospho-αSyn phenotype in vitro, similar to treatment with GCI-purified aggregates [
46]. Identifying exactly which cellular factors affect strain selection is yet to be strictly determined as multiple parameters can influence αSyn conformation.
The cellular environment strongly influencing strain polymorphism is evidenced by the structures of the filaments themselves. As described above, αSyn aggregates isolated from GCIs are composed of two protofilaments arranged around a central cavity [
39]. In this space, coordinated by multiple lysines, are unidentified non-proteinaceous molecules originating from the cell that would influence the packing of said protofilaments. Additional factors may also affect strain conformation, such as post-translational modifications and truncations, which have been shown to significantly modify aggregation kinetics and would presumably lead to the formation of various polymorphs. The previously mentioned study by Rey et al. [
57] includes fibrils formed by C-terminally truncated αSyn, which have more spreading potential and a different distribution pattern compared to the corresponding fibrils made from full-length proteins. This evidence reinforces the notion that it would not be surprising that different patterns of post-translational modifications would generate unique strains. Similarly, molecular chaperones have also been shown to affect fibrillation, with different efficiencies depending on the kinetics of aggregation [
112]. Polymorphs could be differentially repressed based on the prevailing chaperones leading to the selection of specific strains; however, it is still unknown at what stage of the aggregation process the conformational selection occurs.
Because αSyn is intrinsically disordered and folds upon binding to its partners, the selection of conformations is largely influenced by the environment [
113], and it is possible that non-physiological interactions trigger conformational changes that put αSyn on the path of aggregation towards a specific strain. This seems to be the case with the interaction between αSyn and p25α, for example. p25α is a constituent of myelin, possesses a strong affinity for myelin basic protein (MBP), and is commonly located in oligodendrocytes. Notably, in MSA brain tissues, however, p25α re-localizes from the myelin to the cytosol [
114]. p25α colocalizes with αSyn aggregates in cells and induces αSyn aggregation. In vitro, the aggregation of WT αSyn in the presence of sub-stochiometric amounts of p25α leads to the creation of structurally different fibrils compared to WT alone [
115]. It was also determined that treatment of neurons with these αSyn/p25α fibrils led to the creation of larger cell inclusions, while intramuscular injection of the same PFFs reduced the life span of A53T αSyn transgenic (M83) mice and induced increased motor degenerative symptoms compared to WT PFFs [
115].
In continuing with non-physiological interactions influencing αSyn aggregation, the cellular milieu has also recently been noted to play a key role in amplification potential. Although the serial propagation of a strain via in vitro amplified, fibrils achieved the same conformation as the seeds as previously noted [
45,
50], not all environments may be so permissive. It has been established that MSA strains propagate in cell lines expressing either WT or A53T human αSyn [
53] but fail to induce aggregation in E46K αSyn-expressing cell lines. Other interesting observations by Peng et al. [
46] suggest that the cellular milieu can either propagate or modify αSyn strains depending on cell type. First, synthetic αSyn PFFs, when passaged in cellular cultures of oligodendrocytes, neurons, or cortical neurons, acquired distinctive features depending on the cell type. Fibrils passaged in oligodendrocytes showed a superior ability to induce cellular αSyn aggregation, similar to GCI-isolated αSyn aggregates. Then, when the authors propagated LB-isolated αSyn aggregates in transgenic mice that only expressed αSyn in oligodendrocytes, the subsequently isolated aggregates behaved like MSA strains in terms of potency and spreading pattern upon intracerebral injection. The reverse, however, was not true and GCI-isolated strains retained their structural and functional characteristics upon passaging in neuronal cultures.
This idea can be taken one step further, and one could consider that the entry point of the αSyn aggregates, provided they did not arise spontaneously in the brain, could impact the ensuing pathology. The idea that PD, and potentially other synucleinopathies, originate in the periphery remains controversial. First formulated as the ‘dual hit’ hypothesis by Hawkes et al. [
116], the assumption postulates that external factors such as infection or inflammation could induce the aggregation of αSyn in other parts of the body, in particular the olfactory bulb and the gut. This is supported by the presence of LBs in those regions in PD patients. The “gut-to-brain axis” hypothesis gained strong support from observations that severing the vagal nerve prevented the propagation of αSyn pathology in mice models treated with PFFs [
117]. Vagotomy [
118] and appendectomy [
119] could potentially protect against PD development in retrospective cohort analysis, although results remain contentious [
120,
121]. Despite the controversy, multiple routes of entry have been tested—PFFs spread efficiently upon intravenous injection [
54], injection in the duodenum [
122,
123] or the olfactory bulb [
57], for example, but not upon intravitreal injection [
124]. In those cases, the induced pathology mainly mirrors PD. New data from Wang et al. [
125] demonstrated that the accumulation of αSyn aggregates in the detrusor and external urethral sphincter nerves arises not in PD patients but in MSA patients exclusively. They also show in a transgenic mice model that PFF injection in the lower urogenital tract led to the development of αSyn inclusions in the brain—a pattern overlapping the αSyn spread observed in MSA [
125]. Symptoms, including ataxia and paralysis, were also more consistent with MSA than PD. This suggests that PD and MSA might have different origins; the gut for PD and the urogenital tract for MSA. Furthermore, the evidence could also suggest that synthetic PFFs acquire their strain specificity upon cell propagation and that the variations in peripheral neurons influence which strain is ultimately created [
126], as demonstrated in
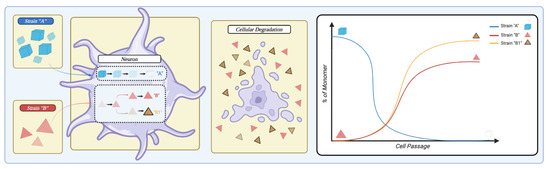
Figure 5.
Figure 5. Representation of the cellular ability to influence strain selection. When a cell is in the presence of two different strains (“strain A” in blue and “strain B” in red), it possesses the ability to influence strain propagation. The cell could preferentially degrade strain A while simultaneously amplifying strain B. Further, the cell can modify strain B to generate a new subtype exemplified by strain B1 in striped yellow. Upon cellular degradation, strains are released that can re-infect a different cell. Overall, multiple cell passages can dramatically alter the landscape of strain representation, as shown in the right panel. Created with
BioRender.com (accessed on 25 June 2023).
This entry is adapted from the peer-reviewed paper 10.3390/ijms241512134