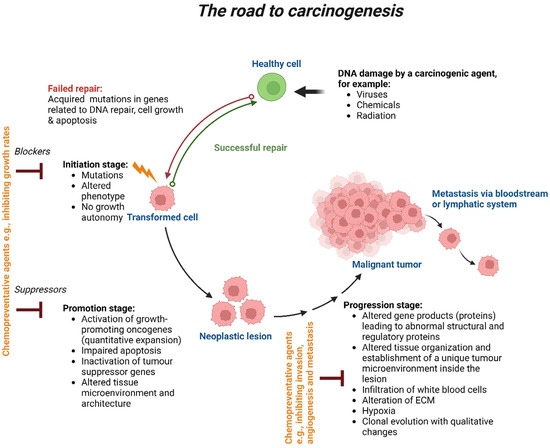
Stages of carcinogenesis. Exposure to a carcinogenic agent, such as viruses, chemicals or radiation, will induce DNA damage in one or a small population of healthy cells. Failure in DNA repair will lead to intrinsic or acquired mutations that will potentially affect the cell’s biological process, such as its growth and apoptosis, resulting in initiating premalignancy transformation. The transformed cells will then promote the growth of neoplastic lesions, which hold so many genetic alterations. Chemopreventive agents, such as natural agents derived from dietary sources (e.g., curcumin, resveratrol) or bioactive molecules (tamoxifen, raloxifene), will be used to block and suppress cell growth rates at those stages to reverse or delay the carcinogenesis process. Failure to eliminate those premalignant cells will lead to the progression stage, resulting in the formation of a malignant tumour. Chemopreventive agents will be given at this stage to inhibit cell invasion, metastasis and angiogenesis. Failure to do so will result in metastasis via the bloodstream or lymphatic system. Created with BioRender.com.
Cancer stem cells (CSCs) exhibit drug resistance because they overexpress adenosine triphosphate (ATP)-binding cassette (ABC) transporters [
16]. Through certain regulatory genes, FOXM1, a transcription factor specific to cell proliferation, controls the transition between the G1/S and G2/M cell cycle phases. Additionally, it is an oncogene that promotes the expansion and multiplication of cancer cells [
17]. Through the expression of ABCC5 (ATP binding cassette subfamily C member 5), FOXM1 overexpression causes paclitaxel resistance in nasopharyngeal carcinoma [
18]. Growth differentiation factor-15 (GDF-15) is a member of the superfamily of transforming growth factor-beta (TGF-β). Proliferation, angiogenesis, stemness, metastasis, drug resistance and immunological modulation are all associated with the overexpression of GDF-15 in cancer. It was demonstrated that stemness and indicators of treatment resistance were significantly positively correlated with GDF-15 expression in breast cancer patients. This suggests that the p-Akt/FOXM1 axis mediates the relationship between increased GDF-15 expression and enhanced stemness and treatment resistance in breast cancer [
19]. Oestrogen receptor positive (ER+)/ human epidermal growth factor receptor 2 positive (HER2+) breast cancer is strongly influenced by the HER2-E subtype and erbB2, which results in resistance to endocrine therapy and a higher probability of recurrence [
20]. About 20–30% of metastatic breast tumours overexpress the human epidermal growth factor receptor 2 (HER2/erbB2), which is associated with a poor prognosis [
21].
3.2. Acquired Drug Resistance
Gradual decline in a drug’s ability to treat cancer after treatment can indicate acquired resistance. A number of factors can contribute to acquired resistance, including changes in the TME following therapy through various mechanisms, such as low pH, hypoxia, shifts and polarisations in the immune cell population, exosomes, various secretomes, vascular abnormalities and soluble factors derived from stromal cells [
11,
12,
31]. Paracrine signalling connections between stromal and tumour cells, mutations or altered levels of drug target expression and activation of a second proto-oncogene that develops into the driver gene, can also contribute to acquired drug resistance [
11,
12,
32].
Targeted medicines cause subtler alterations that can be classified as acquired resistance after repeated exposures or early adaptive responses. Adaptive responses may be the cause of transient clinical reactions because they might happen so quickly that no response is ever clinically evident. Adaptive processes are frequently the result of epigenetic modification and/or non-genetic relief of negative feedback of signalling pathways, which activates parallel pathways or reactivates the initial one [
33,
34].
New genetic mutations can cause resistance and regeneration in cancers that had previously shrunk. Whole-genome sequencing comparing the genetic profiles of eight patients with acute myeloid leukaemia before and after relapse revealed novel gene mutations (e.g., DAXX, DDX41, DIS3, SMC3 and WAC) responsible for tumour resistance and regeneration [
37]. Chemotherapeutic medications disrupt the DNA of malignant cells, which probably accelerates the occurrence of new mutations.
Some non-small-cell lung cancer (NSCLC) patients experience acquired resistance due to circumstances that can interfere with EGFR signalling, such as the upregulation of other RTKs such as MET, the downstream activation of specific pathway elements or phenotypic and histological changes [
39]. Recently, it was demonstrated that EGFR signalling pathways were activated by autocrine EGF and TGF-α and withstood c-Met and anaplastic lymphoma kinase (ALK) inhibition leading to primary and acquired resistance to TAE684/SGX-523 (ALK/c-Met inhibitors) in NSCLC [
40]. In hepatocellular carcinoma (HCC) cells that heavily express c-MET, hepatocyte growth factor (HGF) activated the downstream PI3K/Akt and mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathways through c-MET and concurrently reduced the anticancer effects of lenvatinib (a tyrosine kinase inhibitor) and promoted EMT [
41]. Activating PIK3CA mutations in HER2+ breast cancer will unable a favourable response to pyrotinib plus trastuzumab neoadjuvant therapy [
42]. By encouraging FOXD1 translation through PIK3CA/PI3K/Akt/mTOR signalling, FOXD1-AS1 (an oncogenic long non-coding RNA (lncRNA)) exacerbates gastric cancer development and chemoresistance [
43].
The activation of hypoxia-inducible pathways, EMT, the interaction between the PI3K/Akt and Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathways and the enrichment of tumour-initiating cell population are some of the processes that cause acquired resistance to sorafenib [
45].
3.3. Altered Drug Targets
One of the main causes of drug resistance is drug targeting alteration, which occurs when drug targets’ expression and functionality are altered. Di(2-ethylhexyl) phthalate (DEHP) is a chemical that is frequently found in everyday items and polyvinylchloride medical equipment. As a result, phthalates can enter the human body through eating, inhalation and medical procedures. Phthalates induce cancer progression and chemotherapeutic resistance [
53].
A T315I point mutation that arises in the BCR-ABL kinase domain is the most frequent mutation in BCR-ABL that causes resistance to first-generation (imatinib) or second-generation tyrosine kinase inhibitors (TKIs) that target the BCR-ABL protein, leading to a poor clinical prognosis in chronic myeloid leukaemia [
56,
57]. Due to the activation of intrinsic signalling pathways, such as the RAS/RAF/MAPK/ERK, GSK3 β and JAK/STAT5 pathways, imatinib intolerance or initial resistance arises, and many leukaemic patients acquire secondary resistance [
58,
59,
60,
61].
Moreover, osimertinib is a third-generation powerful EGFR-TKI used to treat NSCLC patients with EGFR mutations. The therapeutic use of osimertinib is nonetheless restricted by the emergence of acquired resistance associated with the triple mutation Del19/T790M/C797S in EGFR [
63].
3.4. Modified Drug Metabolism
Once ingested, drugs are biochemically transformed by drug metabolism enzymes. Metabolic activation is necessary for many anticancer drugs to carry out their mechanism of action. These enzymes have been linked to drug activation and inactivation in cancer cells, including the uridine diphosphoglucuronosyltransferase (UGT) superfamily, glutathione S-transferase (GST) superfamily and cytochrome P450 (CYP) system [
66,
67]. A modification in CYP may alter the capacity of these proteins for drug metabolism, leading to both a large increase in drug release and a change in how the drug is broken down. As a result, patient intratumoural medication concentrations drop, and the treatment loses its effectiveness.
Key metabolic enzymes of 5-fluorouracil (5-FU) include thymidylate synthase (TYMS); 5-FU is a chemotherapy drug and TYMS is one of its target enzymes. It was established that SNHG15 (lncRNA) increased 5-FU chemoresistance in colorectal cancer (CRC) by controlling TYMS expression [
69].
3.5. Enhanced Drug Efflux
The term “drug efflux” refers to the rise in the efflux of cytotoxic medications by active ATP-binding cassette (ABC) transporter proteins, which is one mechanism of drug resistance. Chemotherapy can only be successfully used to a limited extent because of these drug efflux transporters, which lower intracellular drug concentration and inhibit therapeutic response [
72,
73,
74,
75]. Humans have been found to have 48 members of the ABC transporter family. There are only 13 different types of ABC transporters that have been found to play a part in drug resistance in cancer (ABCC1/2/3/4/5/6/10, ABCB1/2/5, ABCA2/3 and ABCG2) [
76]. Breast cancer resistance protein (BCRP/ABCG2), P-glycoprotein (P-gp/MDR1/ABCB1) and multidrug resistance-associated protein 1 (MRP1/ABCC1) are three major ABC transporters that have recently undergone substantial research to better understand how multiple drug resistance (MDR) works [
77,
78].
The role of proteins and signalling pathways in the regulation of ABC transporters in cancer cells has been extensively documented in recent years. Through interactions with upstream and downstream targets, the PI3K/Akt pathway, which is elevated in many human malignancies, has been found to be a critical elusive link in MDR. This signalling pathway promotes the progression of cancer and confers resistance to chemotherapy treatments by increasing the expression of the ABC transporters BCRP, MRP1 and P-gp [
79,
80].
In the human acute lymphoblastic leukaemia cell lines, activation of the MAPK/ERK and JNK pathways upregulated the expression of the ABCB1 and ABCG2 genes, respectively [
81]. Research has also revealed that the ABCG2-mediated multidrug resistance in colon cancer cells is caused by the JNK1/c-jun signalling pathway [
82]
Through the SIRT1/CREB/ABCG2 signalling pathway, miR-132 has also been shown to increase cisplatin resistance in LGR5+ gastric cancer stem-cell-like cells [
84]. ABC transporter proteins are highly expressed on the cell surfaces of CSCs, which have been found to have a significant role in drug resistance and play a role as indicators for CSC isolation and identification [
85,
86,
87,
88,
89,
90].
3.6. Repair of DNA Damage
Numerous chemotherapeutic drugs heavily focus on targeting DNA [
93]. Resistance to drugs that target DNA, however, might be brought on by increased DNA repair and tolerance to DNA damage [
94]. By causing DNA damage, several chemotherapy medicines, including 5-FU and cisplatin, destroy cancer cells. Drug resistance may occur from DNA lesion repairs caused by the DNA damage response of damaged cells to anticancer medications [
95]. The upregulation of p53-target genes on DNA damage response and repair was brought about by 5-FU therapy. When the damage was successfully repaired, the resistant colon cancer cell lines experienced decreased cell cycle arrest and apoptosis, more so than the wild-type ones [
96].
Checkpoint kinase 1 (CHK1) plays a vital role in DNA damage and response and is a crucial effector in the control of replication. The mechanism of resistance to prexasertib, a CHK1 inhibitor, was investigated in sarcoma xenografts [
98]. BCL-xL, an anti-apoptotic protein, was found in higher concentrations and the PI3K and MAPK signalling pathways were phosphorylated more frequently in prexasertib-resistant tumours. Akt, MEK1/2 and ERK1/2 were found to be substantially active in resistant tumours [
98].
3.7. Epigenetics Modifications
Cell destiny and pathogenic provenience are greatly influenced by epigenetics. It appears that non-genetic heterogeneity contributes to the development of cancer-causing cells and/or resistance to treatment. Impairment in gene expression is caused by epigenetic alterations, which last for several cell divisions and finally result in non-genetic heterogeneity and treatment resistance [
105]. The development of chemoresistance in cancer is fuelled by epigenetic changes that are linked to histone modification, DNA methylation, chromatin remodelling and changes associated with non-coding RNA (ncRNAs) [
106]. Accumulating evidence shows that epigenetic changes contribute to the development of various resistance mechanisms, such as improved DNA repair, enhanced drug efflux and defective apoptosis. For example, the chromodomain helicase DNA-binding protein 4 (CHD4), which modulates chromatin remodelling, specifically causes drug resistance in breast cancer gene1/2 (BRCA1/2) deficient cells through aiding DNA damage repair [
107].
Moreover, DNA methylation and gene expression profiles of fulvestrant- and tamoxifen-resistant MCF7 derivatives with oestrogen-responsive MCF7 human breast cancer cells were analysed. Resistance to tamoxifen is developed by significant alterations in downstream ER target gene networks, whereas acquired resistance to fulvestrant revealed a general upregulation of growth-stimulatory pathways, including cytokines and cytokine receptors, the EGFR, ErbB2 and related proteins, the Notch pathway, the Wnt/β-catenin pathway and the interferons (IFN) signalling pathway/IFN-inducible genes, were among the prominently altered pathways in MCF7 cells resistant to fulvestrant [
109].
3.8. Slow Growing Cells
Tumour cells may have transcriptional plasticity, due to epigenetic reprogramming, which will change them into persister cells. These “persisters” are a collection of cells that are slowly growing and have the potential to either re-grow when therapy is stopped or develop enduring resistance. KDM5B, a member of the KDM5A family, designates a small subset of slow-cycling cells in melanomas that are necessary for ongoing tumour maintenance and are dynamically triggered depending on the microenvironmental situation. These KDM5B-positive cells slowly cycle and have increased self-renewal. They are intrinsically resistant to many cytotoxic therapies, and through a dysfunctional Jagged 1/Notch 1-signalling pathway, they can produce offspring that are extremely proliferative [
119].
In response to targeted kinase inhibitors, the histone H3 lysine 27 trimethylation (H3K27me3)-specific demethylases, KDM6A/B, are activated and crucial for the transformation of naive glioblastoma stem cells into the slow-cycling drug-tolerant persisters (DTPs). Pervasive acetylation (H3K27ac) of cis-regulatory components occurs in conjunction with the transition to the persister state and is made possible by a widespread redistribution of the repressive mark H3K27me3. These persisting cells display primitive neurodevelopmental hallmarks because of this modified chromatin state and heavily rely on Notch signalling [
122].
The irreversible stop of cell growth known as cellular senescence is what causes tumour-suppressive pathways regulated by p16 and/or p53 to be activated. As a tumour suppressor, the protein p16
INK4a (also known as p16) inhibits the activity of cyclin-dependent kinases (CDKs) and slows cell division by delaying the transition from the G1 to the S phases of the cell cycle [
124]. Both endogenous and external factors can promote cellular senescence. The three main factors are the shortening of telomere, increased mitogenic signalling created by oncogene activation and non-telomeric DNA damage brought on by chemotherapeutic medicines. Senescence can begin, for instance, when chemotherapy drugs such as doxorubicin and cisplatin cause cell death [
125,
126,
127].
3.9. Undruggable Targets
Several of the most powerful oncogenes and tumour suppressor genes, such as MYC, RAS and TP53, remain intractable despite increasing progress in efforts to target oncogenic driver mutations. Ras proteins were discovered to be oncogenes in the early 1980s, but despite extensive research over more than three decades to identify particular inhibitors, they were thought to be unreachable targets.
In up to 90% of human melanoma, mutated BRAF or mutated NRAS hyperactivate the kinase ERK, according to the examination of genetic changes [
141,
142]. The rationale for developing targeted inhibitors of mutant BRAF and MEK, the kinase that functions downstream of BRAF to activate ERK, as treatments for advanced melanoma was supplied by these findings [
143]. The overall survival of patients significantly increased as a result of the introduction of targeted medicines (MAPK pathway inhibitors such as BRAF and MEK inhibitors) and immunotherapies (immune checkpoint inhibitors). However, a lack of clinical effects, side effects and rapidly escalating treatment resistance limit the long-term efficacy of such treatments. This resistant phenotype is supported by several molecular pathways [
144].
In NSCLC, mutated p53 increases binding to the nuclear factor erythroid 2–related factor 2 (Nrf2) promoter, supported by an activation of the NF-κB signalling pathway, which further increases Nrf2 expression. Nrf2 is a transcription factor that codes for detoxification enzymes and confers resistance to anticancer drugs. In addition, in p53 mutant colon cancer cells, the absence of DNA mismatch repair triggers resistance to cisplatin [
151].
Breast, colorectal, liver and other cancers are all mostly driven by the MYC oncogene. More than 70% of human malignancies exhibit high and/or abnormal Myc expression, which is associated with aggressive diseases and a bad prognosis [
155,
156]. Myc is a difficult oncoprotein to target due to its high frequency of overexpression in malignancies and its pervasive function in transcriptional control. There are presently no specific medications that can be used to target Myc, primarily due to its “undruggable” characteristics: Myc is primarily localised in the nucleus, making it inaccessible to antibodies and lacks an enzyme site where typical small molecules can bind [
157]. BRD4 is a crucial epigenetic regulator (a chromatin regulator) and a member of the BET family. The human genome contains regulatory components, including silencers (repressors), enhancers/super-enhancers and promoters, that are used to dynamically modulate the regulation of transcription. In BET inhibitor-sensitive leukaemia cells, the classic enhancer or super-enhancer controls MYC expression through BRD4 binding. The expression of MYC is inhibited and cell proliferation is suppressed as a result of the BET inhibitor’s blocking of BRD4’s ability to bind to its genomic targets. However, through various mechanisms, long-term drug therapy may restore MYC expression.
3.10. Tumour Microenvironment
Cancer cells, stromal cells, ECM, blood and lymphatic vessels, immune cells, nerve fibres, signalling molecules and related acellular components make up the TME. The latter is sculpted and instructed by cancer cells to support the emergence of cancer hallmarks, react to stimulation, internal or external stress and therapy and eventually support the survival, growth, angiogenesis, migration, invasion and immune evasion as well as drug resistance of these cells [
10].
TME consists of myeloid-derived suppressor cells (MDSCs), mast cells, CAFs, TAMs, vascular endothelial cells, adipocytes, pericytes, tumour-associated neutrophils, dendritic cells and granulocytes. It also includes malignant cells, NK cells and T and B cells. Cancer is protected from immunological eradication by the suppressive immune microenvironment [
4,
163]. Regulatory T (Treg) cells, neutrophils, macrophages, MDSCs, CD4
+, FOXP3
+ and CD25
+ assist in establishing an immunosuppressive pre-metastatic microenvironment [
164,
165]. The activation of MDSCs, TAMs and CAFs by reactive oxygen species (ROS) was demonstrated to be crucial in strengthening their immunosuppressive functions [
166,
167]. Immune cell recruitment into the TME can be affected by the ECM. For example, the ECM can activate the pro-survival pathway PI3K/Akt, which makes it easier for CSCs to evade the immune system [
168]. The recruitment of immunosuppressive cells such as Tregs and TAMs by ECM proteins has also been demonstrated to support CSC survival while inhibiting the recruitment of cytotoxic T cells, which are anti-tumourigenic immune cells [
169,
170,
171].
Key aspects that define cancer stemness, the recruitment of non-malignant cells that support tumour cells and ECM remodelling are coordinated by cellular crosstalk via several signalling networks, such as the juxtracrine and paracrine pathways [
174]. The suppression or modification of interferon-gamma (IFN-γ) signalling, activation of the MAPK and Wnt/β-catenin pathways, a decreased T-cell response and tumour antigen production are a few often found pathways that inhibit the immunotherapy response leading to treatment resistance [
175].
Avoiding detection and eradication by the immune system results in multidrug resistance [
176]. PD-1 is frequently expressed on the membranes of immune cells such as macrophages and T and B cells. While various tumour cells express programmed death ligand 1 (PD-L1). It has been demonstrated that the interaction of PD-1 and PD-L1 on T cell surfaces can inhibit the activity of killer T cells by promoting apoptosis, which causes tumour cells to escape the immune system [
177]. Through the IL-6/STAT3/PD-L1 axis, CAFs modulated neutrophil activation, survival and function in tumour tissues in HCC to promote immune suppression [
178].
MSCs can produce a wide range of cells that engage in paracrine signalling, including IL-6 and IL-8, advancing the development of cancer and enhancing chemoresistance [
179]. When exposed to cisplatin, instead of going through apoptosis, a subpopulation of cisplatin-resistant MSCs activate a phenotype linked to senescence [
179].
In oesophageal squamous cell carcinoma, PAI-1 secreted by CAF activate the MAPK and Akt pathways in a paracrine manner resulting in the production of ROS and the induction of DNA damage and cell death leading to chemoresistance [
182]. Additionally, drug resistance was promoted in tumour cells via NF-κB pathway induction by CAF-derived paracrine signals, such as exosomes, metabolites and chemoattractant cytokines [
183,
184]. CAFs can also enhance stemness through NF-κB signalling activation in gastric cancer [
185].
Depending on their location within the cancer tissue, the cells in the tumour mass grow in a 3D tissue structure and are unevenly exposed to oxygen. As opposed to the tumour core, which is poorly vascularized, blood vessels in tumour tissues are typically randomly arranged and only cover the outer portion of the tumour mass [
189]. A hypoxic microenvironment is created within the tumour core as a result of increased tumour cell proliferation, which places the cells there further away from the supporting blood vessels than the cells outside the tumour.
The TME causes chemotherapeutic resistance via intrinsic or acquired mechanisms. Cancer dormancy, stemness and progression, as well as intercellular communication, redox adaptability and drug resistance, are reprogrammed by hypoxia [
195]. Hypoxia affects the TME and treatment efficacy by encouraging cancer cells’ greater production of hypoxia-inducing factors (HIFs), most frequently HIF-1α. This latter stimulates the transcription of numerous genes, including vascular endothelial growth factor (VEGF), which enhance angiogenesis and, as a result, cancer cells are better able to sustain their oxygen supply and metabolism, improving their chances of surviving [
196,
197].
One of the characteristics of cancer is metabolic reprogramming, which is a modification in metabolism or nutrition supply. Increased metabolism of glutamine, glucose, amino acids, lipids, addiction to ROS and accumulation of lactate are common characteristics of cancer [
201,
202,
203]. The synthesis of brain-derived neurotrophic factor by CAFs was driven by lactate in cancer cells in an NF-κB-dependent way, which in turn activated TrkB/Nrf2 signalling in cancer cells to lessen their susceptibility to anlotinib [
204]. These results support the connection between drug resistance, metabolism and NF-κB signalling.
Cancer is characterized by dysregulated pH, which is one of the TME variables. Extracellular pH (pHe 7.3–7.5) is often higher than intracellular pH (pHi 6.8–7.2) in healthy tissues and cells, while cancer cells generate a “reversed pH gradient” with increased internal pH and decreased external pH [
205,
206,
207]. This reversed pH gradient makes it difficult for cancer cells to undergo apoptosis and prevents them from dying off [
208,
209]. Cancer cells’ acidic extracellular environment (pH 6.5–7.1) plays a role in their chemotherapy resistance [
210].
Neurology and cancer sciences are closely related, with neurotransmitters and neuropeptides generated from the nerve creating an “innervated niche” [
219,
220]. The neuroligin-3 (NLGN3)-stimulated PI3K/mTOR pathway, which is activated by active neurons, aids in the formation of high-grade gliomas [
221]. Paracrine stimulations of cGAMP to astrocytes, cytokines production, the activation of the STING pathway and NF-κB and STAT1 signalling are triggered in brain metastatic cells via gap junctions between astrocytes and lung/breast cancer, which promotes cancer growth and resistance to chemotherapy [
222,
223].
The creation of a mechanical niche depends on stromal cells, extracellular and intracellular components and intercellular signalling [
224]. There are various structural proteins in the ECM such as collagen, laminins, fibronectin, elastin, proteoglycans and glycoproteins. The ECM is a 3D network of macromolecules that provides the biochemical and biophysical characteristics of the non-cellular bulk surrounding the cells. Additionally, non-malignant tumour-associated stroma cells are a crucial component of the TME, altering tumour characteristics, illness prognosis and therapeutic response. Cell surface proteoglycans, cell adhesion molecules such as integrins, and hyaluronic acid receptors such as CD44, mediate biochemical and biophysical signalling as well as cell anchoring to the ECM [
189,
225].
Matrix cells in the TME communicate with cancer cells through exosomes. Exosomes are small, bilayered molecules involved in autocrine, paracrine and endocrine signalling that are released by stromal and cancer cells in the TME. Altering vital survival signal transduction pathways, inducing EMT, activating anti-apoptotic pathways and modifying the immune system are just a few of the ways that exosomes can make tumour cells resistant to treatment [
229]. The exosome-mediated transfer of different ncRNAs, such as lncRNAs and miRNAs, may be a way for cancer cells to develop treatment resistance by causing genetic and epigenetic changes [
229,
230].
3.11. Epithelial-Mesenchymal Transition
The phenotypic change from epithelial to mesenchymal cells, or epithelial-mesenchymal transition (EMT), occurs when epithelial cells lose their cell identity and take on mesenchymal traits, altering the cell’s shape and expression of surface markers in the process [
236]. Epithelial cells, in the EMT process, experience depolarization, lose their cell-cell contact and adherent property and develop elongated fibroblast-like morphology, which is known to be triggered by ncRNAs, growth factors, cytokines and hypoxia. These occurrences are accompanied by a concurrent increase in mesenchymal markers (integrin, laminin 5, N-cadherin, fibronectin, vimentin and type I collagen) and a concurrent decrease in epithelial markers (laminin 1, desmoplakin, E-cadherin and type IV collagen) expression. EMT is typically seen under healthy conditions, but tumour cells have the ability to carry out the same process while cancer is developing. Recent evidence suggests that pathological hyperactivated EMT is closely linked to elevated therapeutic resistance in cancer cells. Intracellular regulatory miRNA, exogenous inducers, epigenetic modulators and cellular signalling pathways such as SMADs, PI3K, MAPK, ERK, TGF-β, Notch and Wnt/β-catenin are only a few of the molecular players involved in the regulation of EMT [
237,
238].
3.12. Multidrug Resistance
MDR is a common problem in cancer patients undergoing long-term chemotherapy and is the primary cause of death. Some tumours that become resistant to one type of drug are also found to be resistant to different drugs, despite the fact they might have different modes of action from the primary therapy. In fact, cross-resistance to a variety of anticancer medications with unique structural and functional characteristics is a hallmark of the MDR phenotype. As discussed in the sections above in more detail, host factors, tumour factors and tumour-host interactions are just a few of the many variables influencing drug resistance, but also MDR.
Genetic variations and drug-drug interactions are examples of the hosts’ contributing elements. Genetic variants, such as single nucleotide polymorphisms (SNPs), copy number variations, insertions, deletions and repeats in genes encoding drug targets, DNA repair, cell cycle control, drug efflux and enzymes that are related to drug metabolism, can affect drug efficacy [
12]. Drug-drug interactions can change drug efficacy by interfering with the drug’s pharmaco-kinetics and -dynamics when the cancer patient, at the same time of their treatment, is administrated another drug, herbal supplement or is exposed to an environmental factor (e.g., diet, smocking, exposure to chemicals) [
251].
4. Altered Signalling Pathways Involved in Drug Resistance to Cancer
4.1. Wnt/β-Catenin Pathway
It has been discovered that EMT and resistance to chemotherapeutic drugs in cancer cells depend on the zinc-finger transcription factor pleomorphic adenoma gene like-2 (PLAGL2). Recently, it has been demonstrated that through the activation of the Wnt/β-catenin signalling pathway, PLAGL2 encourages adriamycin resistance and the aggressiveness of cells in breast cancer [
254]. A recent study on squamous transitioned lung cancer suggested that Wnt signalling may have a role in increasing adeno-to-squamous transdifferentiation (AST) [
255].
4.2. The JAK/STAT Signalling Pathway
It was discovered that miR-106a-3p is an oncomiR in gastric cancer that triggers apatinib resistance due to the overexpression of JAK2/STAT3 proteins and their signalling [
260]. Moreover, through the PTEN/Akt/SMAD2 and RAS/MEK/FOS MAPK/Akt pathways, the apurinic/apyrimidinic endodeoxyribonuclease 1 (APEX1)/miR-27a-5p axis contributed to the resistance to doxorubicin in gastric cancer cells [
261].
4.3. PI3K/Akt/mTOR Pathway
Proprotein convertase subtilisin/kexin type 9 (PCSK9), a crucial enzyme for antitumour immune responses, also activated Akt by suppressing PTEN, which caused HCC to develop sorafenib resistance [
263]. Furthermore, nuclear paraspeckle assembly transcript 1 (NEAT1), which is a lncRNA, activated the c-MET/Akt pathway via miR-335 in HCC cells, resulting in sorafenib resistance [
264].
4.4. MAPK Pathway
Recently, it was demonstrated that mitochondrial fusion dramatically decreases the susceptibility of breast cancer cells to tamoxifen under metabolic stress and likely contributes to the development of acquired drug resistance through AMPK and MAPK signalling [
268]. Through p44/42 MAPK-Drp1 (a dynamin-related GTPase) signalling, membrane-bounded G-protein coupled oestrogen receptor (GPER) causes mitochondria fission, which is essential for GPER-induced cell apoptosis in breast cancer cells [
269]. According to pertinent studies, one of the key mechanisms by which CRC cells develop resistance to cetuximab is the activation of the RAS/RAF/MEK/MAPK pathway [
270]. Fucosyltransferase VI (FUT6) modulates the EGFR/ERK/STAT signalling pathway to control head and neck squamous cell carcinoma invasion, migration, proliferation and EGF-induced EMT [
271].
5. Strategies to Overcome Drug Resistance in Cancer
5.1. Circulating Tumour DNA
Liquid biopsy has received a lot of interest in oncology diagnosis over the past several years. It is a biological sample approach that offers details on the real-time dynamics of tumour biomarkers in a quick, cheap, easy to access, minimally invasive and patient-friendly way. Several soluble components associated with tumour genetics include exosomes, circulating tumour cells, cell-free DNA (cfDNA) and circulating tumour DNA (ctDNA). In the latter, genetic modifications associated with cancer can be detected, such as amplification, point mutations, aneuploidy, rearrangements and patterns of fusion and methylation. By using platforms based on the polymerase chain reaction (PCR) and next-generation sequencing (NGS), liquid biopsy can be analysed to depict the current complexity of the patient’s overall tumour mass [
273,
274]. ctDNA amount may serve as a prognostic indicator as its analysis may reveal the factors affecting prognosis. Following surgical resection, ctDNA is sensitive enough to detect minimal residual disease (MRD). Following surgery, ctDNA analysis offers a good prognostic evaluation and can help identify patients who have a very high risk of recurrence, potentially avoiding unnecessary chemotherapy.
5.2. Immunotherapy
Moreover, immunotherapy, which includes cancer vaccines, monoclonal antibodies and inhibitors of immune checkpoints such as anti-PD-1/PD-L1 and anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA4) [
275,
276], is promising in that it may revolutionise the treatment of cancer by inducing, enhancing or suppressing immune responses against cancer cells. Recently, anti-CD47 agents have gained attention. Numerous tumour cell surface membranes have high levels of CD47 expression, which controls macrophage phagocytosis by binding SIRPα to prevent the eradication of host cells. Inhibiting the interaction between cancer cells and macrophages and inducing phagocytosis may be achieved by CD47-blocking drugs, such as monoclonal antibodies that target CD47/SIRPα [
277]. Thus, combining immunotherapy and chemotherapy can be an effective approach to overcoming drug resistance. For example, recent clinical trial results for unresectable HCC have shown that combination therapies, such as tremelimumab (anti-CTLA4 Ab) (HIMALAYA) + durvalumab (anti-PD-L1 Ab) + bevacizumab (anti-VEGF Ab) (IMbrave 150) + atezolizumab (anti-PD-L1 Ab) outperform monotherapy in terms of clinical outcomes [
278,
279].
5.3. Nanotechnology
Nanoparticle-based medications have effectively decreased side effects, eliminated drug resistance and increased medicinal efficacy [
286]. The selectivity of the target gives nano-based medications an edge over traditional therapy [
287]. Numerous nanoparticles, including mesoporous silica, metal and polymeric nanoparticles, as well as micelles, liposomes, dendrimers and nanostructured lipid carriers, have been created and investigated over time and have significantly reduced chemoresistance in cancer [
288]. The newly created doxorubicin-melittin polymersome (Dox-Mel PL) drug delivery system was capable of controlling MDR cancer cells and offered the following benefits: (1) biocompatible polymersome (a poly lactic acid-hyaluronic acid (20k–10k) di-block copolymer) promote synergistic effects of the simultaneous administration of Dox (anticancer agent) and Mel (a major component of bee venom); (2) reduction of Dox and Mel side effects; and (3) downregulation of P-gp by Mel prevent drug resistance. Dox-Mel PL overcomes MDR through P-gp inhibition and PI3K/Akt/NF-κB pathway downregulation [
289].
5.4. Gene Editing
It is common practise to utilise high-throughput forward genetic screening methods to investigate the molecular processes behind particular cellular phenotypes, such as treatment resistance in malignancies. To undertake loss-of-function screening across a variety of signalling pathways and biological processes, CRISPR-associated nuclease Cas9 (CRISPR/Cas9) is a particularly successful method. Single or double knockouts or the modification of genes responsible for drug resistance can now be produced using the genome-wide CRISPR/Cas9 gene editing method [
296,
297].
5.5. Computational Strategies
Moreover, the emergence of deep learning, the vast amount of digital data and powerful computing resources can offer an effective pipeline for enhanced drug discovery, help us understand how drugs become resistant to them and help us make the best decisions possible when treating patients with EGFR-mutated NSCLC [
299,
300]. Furthermore, Fröhlich et al. recently discussed the second-generation MAPK Adaptive Resistance Model (MARM2.0), which aims to explain how drug-adapted BRAFV600E melanoma cells rewire EGFR/MAPK signalling. MARM2.0 is developed using rule-based modelling in PySB (python program) with thermodynamic balance and builds on an extensive body of theoretical, biochemical and structural work on EFGR/MAPK signalling and feedback regulation [
301].
5.6. miRNAs
miRNAs can also control drug sensitivity and modulate resistance by post transcriptional gene regulation. Therefore, they do not only serve as biomarkers, but also as drug targets for overcoming drug resistance. For instance, exosomal miR-107 modulated the HMGA2/mTOR/P-gp axis, drastically increasing the susceptibility of resistant gastric cancer cells to cisplatin, indicating that exosomal miR-107 may be a promising target in the therapy of gastric cancer [
304].
5.7. Targeting Signalling Pathways
PTK overexpression, including HER2, EGFR, FGFR, PDGFR, VEGFR and IGFR, activates numerous cell signalling pathways, including STAT3, NF-κB, PI3K/Akt and ERK1/2. It also results in an aberrant expression of proteins associated with apoptosis in cancer cells, which is a major contributor to chemotherapy resistance in tumour cells. Target therapy directed at specific tyrosine kinases will therefore overcome this resistance. There are various instances where platelet-derived growth factor (PDGF) ligands and receptors are both expressed in malignant cells; nevertheless, PDGF expression and function typically involve the tumour stroma. The pursuit of PDGFR inhibitors represents a successful strategy given the significance of the TME and the critical part that PDGF signalling plays in creating and maintaining that milieu [
307].

 Encyclopedia
Encyclopedia
