Prevailing as the most threatening cancer affecting females, BC has been a major health concern for more than a decade now, with 2012 GLOBOCAN statistics registering a staggering 18% rise in casualties compared to the number of 2008 cases [
165,
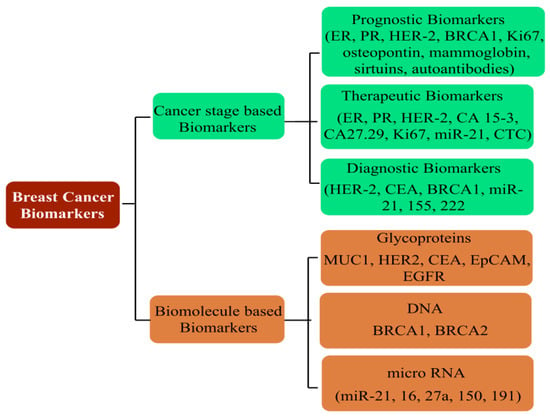
166]. A predictive analysis by the American Cancer Society anticipates one out of every eight United States females to develop BC in her lifetime. On a global platform, too, trends reflect a significant concern, with an addition of 3.2 million new cases per year until 2050. As rising mortality concerns continue unabated, it is a priority to screen accurate and reliable biomarkers, in addition to timely diagnosis, to improve the success of preventive treatment (
Figure 6). Development in diagnostic assays in the past few years has simplified the treatment rigor, whereby rising mortality has witnessed a substantial arrest. Global awareness campaigns and recent research findings have enhanced the unanimity for BC classification, vis-à-vis characteristic histology and molecular events.
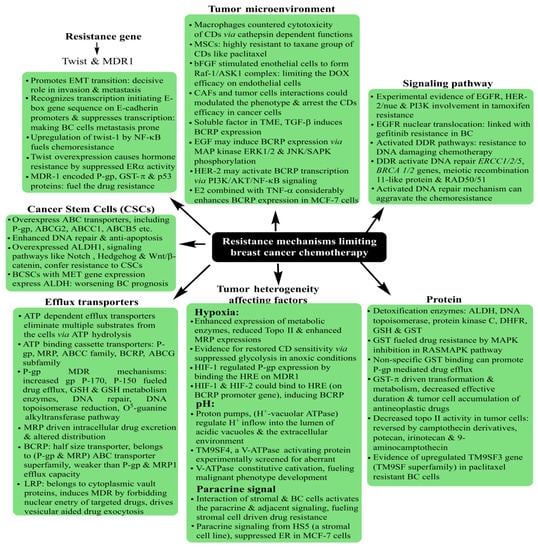
Familiar histological subtypes identified to date include medullary, metaplastic, apocrine, mucinous, cribriform, tubular, neuroendocrine, classic lobular and pleomorphic lobular carcinomas, in addition to the non-specific invasive ductal regimes that characterize the majority of recently screened cases. From the molecular viewpoint, the classification is made via the expression of distinctive surface receptors, categorized as estrogen/progesterone (ER/PR)-positive, negative and triple-negative (no ER/PR and HER2). The ER-positive tumors are rather more common, with a smaller size, lower grading and being lymph node-negative. In a nutshell, Luminal A and Luminal B constitute the ER/PR-positive subgroups, while the ER/PR-negative tumors are distinguished as HER2 (Human Epidermal Growth Factor2), basal and normal subtypes [
3,
4]. Amongst all the subtypes, the triple-negative (TNBC) state (no ER, PER and HER2) exhibits the most aggressive pathology, contributing to ~10–20% of BC deaths. The lack of positivity of the surface markers renders the TNBC non-treatable with targeted therapies.
The therapeutic success of tumor treatment is substantially affected by its discrete recognition, accomplished via characteristic OSRs, a summary of which is described in Table 1. For instance, the HER2-overexpressing BC has a higher probability of being implicated in inflammatory responses, with prominent involvement in angiogenesis, lymphangiogenesis and aromatase upregulation. Similarly, toll-like receptors (TLRs) are firmly associated with adhesion and invasion via modulating αvβ3 integrin expression, attenuated TLR-4 activity resulting in reduced IL-6, 8 actions, alongside the TLR-3-mediated inhibition of cell proliferation and survival. These actions of overexpressed surface receptors could be the basis of therapeutic mechanisms in targeted therapies, moderating the stress of excessive CD intake via exercising the local therapeutics on improved tumor cell internalization.
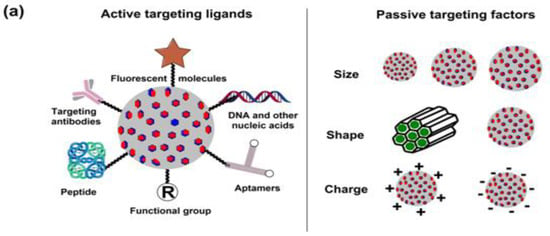
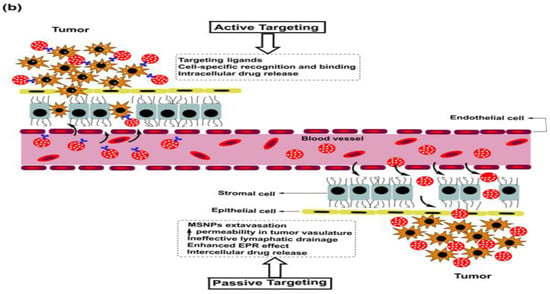
A thorough understanding of overexpressed surface receptor chemistry vis-à-vis ligand specificity could enhance the success of drug delivery to BCs, wherein the advanced stages are often characterized by metastasis to lungs and the brain. Ligand variation is indeed a challenging prospect in this domain, as stoichiometry concerning the optimum drug extent for therapeutic success is a daunting challenge considering the troubled pathophysiology in the tumor vicinity. The versatile features of MSNPs with tailored surface chemistry, varying pore sizes, functionalization diversity and morphology-tuned drug adsorption make them unique and capable of tuning receptor–drug interactions and enhancing tumor cell internalization.
This section discusses the major findings of recent studies (2017 onwards) using MSNPs for BC treatment, distinguished via singular and dual therapeutic agents. Emphasis has been laid on comparative IC50 reductions, moderated drug release kinetics and enhanced tumor cell internalization abilities. The advantage of dual therapeutic agents is exhibited by the stoichiometric variation of a combinatorial mode, involving the structural changes that decrease the chances of obtaining a resistant response. Although MSNPs do prevail as a carrier in both modes, the therapeutic efficacy of a combined mode is generally higher (likely due to a synergistic influence), owing to which substantial IC50 reductions could be accomplished.
Commencing from 2017, the first major effort used polydopamine (PDA) and polyethylene glycol (PEG)-coated MSNPs for DOX delivery to MCF-7 and MDA-MB-231 BC cells. The PDA coating enabled a pH-mediated DOX release, while the PEG functionalization conferred an improved physiological biocompatibility. Dispersion profile analysis for the MSNPs in neat, DOX-loaded, PDA and PEG coated states, revealed increments and decrements in PS and PVs. Though PDA and PEG-coated MSNPs exhibited pore sizes of ~200 nm, which were smaller than the cut-off limits of tumor neovasculature pores and exhibited suitability for the EPR effect. Anionic surface sensitivity guarded against reticuloendothelial clearance, facilitating an elongated physiological residence. The inspection of the morphology using Transmission Electron Microscopy (TEM) implied a spherical morphology with porous surfaces for all states of the MSNPs, conveying the structural compatibility of PDA and PEG coating. These physicochemical features together contributed to a ~95% DOX encapsulation efficacy (EE). An analysis via confocal microscopy and flow cytometry for cellular uptake distinctions revealed enhanced extents for PDA and PEG-coated, DOX-loaded MSNPs compared to free DOX. These observations were supported by the MTT assay, which implied higher cell growth and proliferation inhibition for PDA and PEG-coated, DOX-loaded MSNPs than free DOX and the NPs without PEG coating. The antitumor mechanism of intact MSNPs was screened using fluorescence analysis, wherein MSNP–DOX–PDA–PEG-treated MCF-7 cells formed greater autophagic vesicles (green fluorescence) over other configurations. The regulatory influence of the autophagic response was screened via assessing the AKT, mTOR and p70S6K phosphorylations, with MSNP–DOX–PDA–PEG causing the maximum inhibition. The observations were replicated in the nude mice harboring subcutaneous MCF-7 tumors, with MSNPs–DOX–PDA–PEG exhibiting the maximum inhibition in terms of maximum tumor weight decrements. Furthermore, no significant distinctions in the body weight and histopathological abnormalities (heart, liver, spleen, kidney and lungs) for all the carrier configurations imply the biocompatibility of MSNP–DOX–PDA–PEG [
169]. This study, therefore, optimized the pH-specific DOX release kinetics via PDA and PEG–MSNP functionalization, conferring high tolerability with enhanced tumor cell uptake.
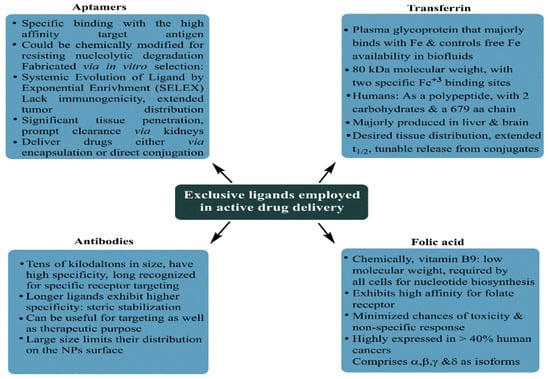
A subsequent effort in 2017 used folic acid (FA) and N-acetyl glucosamine (NAG)-functionalized MSNPs for DOX delivery to MCF-7 and MDA-MB-231 human BC cells. With a 78 nm PS and a cationic surface sensitivity (unlike the previous study), the prepared MSNPs exhibited compatibility for EPR-driven tumor cell internalization aided by electrostatic receptivity with negatively charged membrane lipids. Monodispersed states with the spherical morphology of prepared MSNPs were inferred by Scanning Electron Microscopy (SEM) analysis, while TEM screening implied their sieve-resembling structure, harboring nanoporous channels on the surface. No structural abnormality was observed for FA and NAG functionalizations in the FTIR screening, but a thermal stability inspection using thermogravimetric inspection revealed a 10% and 19% higher weight loss for NAG and FA-functionalized MSNPs compared to their neat state. This weight loss was attributed to the moderated NAG and FA contributions, with nearly twice the FA molecular weight contributing to its higher loss. Cellular uptake studies using confocal microscopy and fluorescence spectroscopy (comparative red and green intensities) both implied the significant uptake of NAG and FA-functionalized, DOX-loaded nanocarriers (NCs). Quantitative screening by flow cytometry revealed 52.36 ± 3.98 and 60.1 ± 9.37% internalizations for FA and NAG-functionalized NPs in MCF-7 cells, while similar amounts for MDA-MB-231 cells were 52.74 ± 1.08 and 60.58 ± 6.03%. The ligand conjugation of DOX-loaded NCs enhanced their toxicity, as screened by the MTT assay, with 48 and 72 h viability for 100 μg·mL
−1 extents being 10.68 ± 0.59 and 4.34 ± 0.45% for DOX–NAG–MSNPs and 14.57 ± 0.65 and 8.95 ± 1.61% for DOX–FA–MSNPs. Similar extents for more aggressive MDA-MB-231 cells were 5.41 ± 0.12 and 5.15 ± 0.41% for FA-functionalized and 7.90 ± 1.67 and 5.76 ± 0.31% for NAG-functionalized DOX-loaded NPs. The NAG functionalization exhibited greater cytotoxicity (IC
50 = 0.86 μM) than FA (IC
50 = 2.5 μM), which was explained by investigators to be due to a comparatively higher glucose transporter (GLUT) expression on BC cells’ surface compared to the folate receptor α (FRα). The mechanism of the anticancer response was screened using AO/EB fluorescent staining, with minimal necrotic cells exhibiting varied orange-red EB fluorescence, while AO penetrated the intact membrane cells and developed green fluorescence. These distinctions implied apoptosis to be the likely source of cell death following exposure to drug-loaded MSNPs [
170].
6.2. Combinatorial Drug Delivery Using Mesoporous Silica Nanoparticles
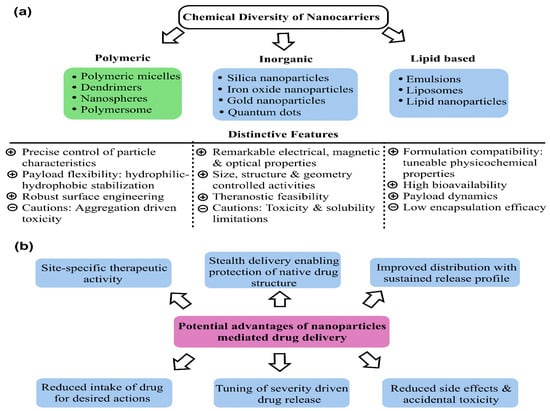
Recurrent resistance with CDs has reduced their clinical efficacy, thereby mandating a need for shifting to potential alternatives via predictive structure–activity relationships (SARs). In this regard, the combinatorial delivery of CDs (two or more) with sensitization agents (such as photothermal or X-ray irradiation) has lately gathered significant interest. The turning point aspect revolves around the long-known and practiced status of most of the CDs due to which their continued use inevitably receives a resistant response despite a stealth delivery via various NCs. To counter the competing therapeutic limitations, combining two or more CDs or the sensitizing agents with CDs has lately been an area of focus for developing potent drug delivery vehicles. This strategy benefits from multiple considerations, wherein the most important distinction is the reduced elimination on an in vivo scale. Newer combinations are not recognized as native, unlike the long-practiced CDs, whereby there stands a significant possibility of these being more effective. Secondly, for terminally ill or advanced stages (as in TNBC), rapid metastasis and fragility mandate accurate therapies. Using the combinatorial route, the concentration of the major CDs (often resistant) is effectively reduced, wherein the sensitization of healthy cells is substantially reduced. The combination of drugs no longer targets a single receptor on tumor cells and its unique chemical essence is the tunability with the mutated status of receptor proteins. Thereby, the chances of prompt elimination from physiological boundaries are much lower for a combinatorial regime. The most important benefit of combinatorial delivery is the possible use of bioactive and non-toxic nutraceuticals. Therefore, delivering a small proportion of CDs with these compounds could significantly improve their tumor cell internalization. Stronger therapeutic essence and dosage moderation are inevitable via NC-mediated delivery, as additional structural protection is accomplished. As for MSNPs, recent interest has superseded the success of Au and Ag NPs, both of which lack the porous morphology, and Ag NPs also exhibit high native toxicity that complicates their systemic elimination. Keeping these prospects in the background, the recent attempts (since 2017) using MSNPs for combinatorial drug delivery to treat BC are summarized in the following paragraphs.
The single significant attempt from 2017 used TiO2-coated MSNPs as DOX carriers, with the assistance of 808 nm near-infrared (NIR) irradiation for thermal imaging-aided photothermal therapy (PTT) for BC chemotherapy. The study by Ren and colleagues highlighted the constraints of traditional white TiO2-like weak drug loading, inadequate UV light penetration in the tissues and the heating risks of 980 nm NIR to the healthy tissues. The preliminary screening of black TiO2 (B-TiO2) NPs revealed a PS of 25 nm (via TEM) with weak dispersion. Coating these NPs with mesoporous silica conferred a core–shell morphology to B-TiO2 NPs with a ~100 nm diameter. The prepared nanocomposites (B-TiO2 NPs in an MSNP core) were functionalized by the -NH2 group and subsequently reacted with-COOH in the FA, using an EDC–NHS conjugation. The recovered NCs were loaded with DOX, exhibiting a 150–260 nm PS and a <1 PDI for B-TiO2, NC, NC–NH2, NC–FA and NC–FA–DOX. The –NH2, FA and DOX conjugation to the NC surface was distinguished via the cationic surface sensitivity for B-TiO2 and NC–NH2comparedto anionic sensitivity for others. The DOX loading on NCs was screened as 5%, ~10-fold higher than uncoated B-TiO2. Screening the photothermal irradiation impact on DOX release from NCs, NIR irradiation at pH 5 enhanced the release from 60.6% (neat) to 91.3% in 48 h, conveying a pH and NIR-modulated liberation. The impact of FA targeting was distinguished in the fluorescence analysis, wherein NC–FA–DOX exhibited DOX deposition in the nucleus, while the non-targeted NCs (without FA) revealed little DOX in the cytoplasm, with inadequate nuclei localization. Screening the PTT influence on the therapeutic efficacy of DOX, a better performance for NC–FA was noticed than for NCs alone, with a 0–5 min NIR exposure on incubation with 75, 150 and 225 μg·mL−1 Ti, generating the 85.2 and 50.8% at 75 μg·mL−1, 76.5 and 10.9% at 150 μg·mL−1 and 34.1 and 5.5% at 225 μg·mL−1 viabilities for NC and NC–FA confirmations.
Monitoring the influence of PTT on the anticancer effects of DOX, the analysis revealed cell death of 31.6% (by DOX alone) and merely 8% (by NC–DOX); and with NC–FA–DOX, this extent was 28.7%. On subjecting the treated cells to 5 min NIR irradiation, these limits increased to 44.8, 34.1 and 93.8%, implying the synergism of PTT and chemotherapeutic treatments. Screening the cell death mechanism in NC–FA and NC–FA–DOX-treated cells, a flow cytometry-driven inspection revealed most tumor cells being subjected to necrosis (death via photothermal-induced ablation). The in vivo screening of chemotherapy and PTT synergism was made in tumor-implanted xenograft mice, distinguished as (i) only DOX-treated, and (ii) treated with NC–FA, (iii) NC–FA + NIR irradiation, (iii) NC–FA–DOX and (v) NC–FA–DOX + NIR irradiation. An analysis of five mice, which were sacrificed to consolidate the PTT–chemotherapy synergism using HE staining revealed most cells to be dead in the DOX, NC–FA + NIR, and NC–FA–DOX groups, exhibiting nuclear or cell membrane damage. Greater tumor cell killing in the NC–FA–DOX + NIR group suggested a synergistic combination of PTT and chemotherapy rather than a singular effect. The observations were further strengthened by significant decrements in TV for the NC–FA–DOX + NIR group compared to the NC–FA + NIR and NC–FA–DOX groups. Moreover, no significant variations in body weight revealed the biocompatibility of the used NCs [
192]. Thereby, this study established a synergism of TiO
2 NPs’ toxicity with the FA-conjugated MSNPs for the localized therapeutic induction in the MCF-7 BC cells. Similar attempts could be made by co-delivering TiO
2 NP-doped MSNPs with other CDs and some phytomolecules, which could further moderate the toxicity. Secondly, in place of Ti, the oxides of other elements (in the same group of the periodic table) could be a logical extension of this study.
The year 2019 reported a sole major attempt relying on HER2-conjugated aptamer (HApt) cytotoxicity via cross-linking and subsequent HER2 translocation to cytoplasmic vesicles in the SKBR3 and MCF-7 BC cells. Relying on HER2 suppression-triggered impaired cell proliferation and impaired apoptosis, Shen and colleagues exploited the HApt targeting and antagonizing ability to augment the HER2-positive BC cell-specific toxicity of MSNPs. The carrier configuration involved HApt-functionalized pH-sensitive β-cyclodextrin (β-CD) capped with MSNPs conjugated with benzimidazole (MSN-BM), which was used to optimize a pH-driven DOX release. The purpose of including β-CD was to use its gatekeeper essence to deliver encapsulated DOX-HApt as the HER2-targeting biotherapeutics. The thorough physicochemical characterization of the optimized carrier revealed nanoscale attributes (via FT-IR, XRD, TEM and BET) with no structural arbitration. The DOX release was studied at pH 7.4, 6.4 and 4.5, with the maximum extent (>80%) being accomplished at pH 4.5, due to BM protonation-disrupted DOX–BM hydrophobic interactions. Comparing the NC-delivered DOX efficacy in HER2-positive SKBR3 and HER2-devoid MCF-7 cells, greater cytotoxicity, as well as enhanced uptake, was observed in the SKBR3 cells. The synergistic association was witnessed as the DOX-HApt co-delivery enabled higher toxicity in HER2-positive BC cells compared to either DOX or HApt. The reduction in cell viability for DOX-devoid NCs was15 ± 2%, for 100 μg·mL
−1 NPs, while similar results for free DOX and aptamer-devoid NC–DOX (DOX extent: 3.6 μg·mL
−1) were 8 ± 4% and 27 ± 6%. The MSN-BM/CD-HApt@DOX, however, reduced the viability by ~68 ± 6%, signifying a DOX and HApt synergistic essence [
193].
The first major 2020 attempt focused on MSNP-facilitated combinatorial drug delivery to treat BC, using lactoferrin (Lf)-coupled NPs for simultaneous pemetrexed (PMT, a cytotoxic drug) and ellagic acid (a phytocompound) administration to MCF-7 human BC cells. The hydrophobic EA was physically encapsulated within the mesopores (via adsorption) assisted by electrostatic interactions between anionic EA and -NH
2 group functionalized NCs. Contrary to this, the hydrophilic PMT was chemically conjugated to Lf coating via carbodiimide coupling, to prevent its early release and instantaneous toxicity aggravation. The dispersion analysis of PMT + EA-loaded NCs attributed their nanoscale distinction, with a PS of 284 nm (via DLS), 190–230 nm (via TEM imaging) anda 0.207–0.778 PDI, enabling a sequential drug release, first of EA, followed by sustained PMT liberation. The co-loading features were demonstrated well via differential scanning calorimetry (DSC) and powder XRD, showing a simultaneous drug loading that resulted in crystallinity loss. Structural coherence was inferred by FTIR spectroscopy with C=O ester of EA (1723 cm
−1), C=O functionality of the second –COOH group of PMT (1690 cm
−1), and the (1690–1630 cm
−1) stretching frequencies of newly formed amide linkages. The synergistic association between PMT and EA was monitored via cytotoxicity studies, wherein 5:3 proportions of unaided EA and PMT exhibited 1.7 and 1.3-fold decrements in IC
50 of EA (34 μg·mL
−1) and PMT (26 μg·mL
−1), complemented by a combination index (CI) of 0.975. These observations were further strengthened for NC-mediated EA delivery with a 23 and 20 μg·mL
−1 IC
50 for non-targeted and targeted NC configurations. Yet again, a CI value of 0.885 suggested EA and PMT synergism (less so than for unaided delivery). The role of Lf targeting in desired drug internalization in the MCF-7 tumor cells was screened via fluorescence intensity, which developed a faint green appearance, inhibiting the Lf receptors and incubating the cells with excess Lf before the uptake analysis. The results were supported by a flow cytometry inspection, wherein high MFI implied a higher intake of NCs by the tumor cells in the Lf-conjugated state [
194]. Thereby, this study established Lf-targeted MSNPs as promising carriers for mediating synergistic PMT and EA anticancer actions in MCF-7 BC cells. The results indeed encourage evaluation in animal models and more aggressive BC subtypes.
A subsequent 2020 attempt towards using MSNPs for combinatorial therapies against BC involved DOX and a sonosensitizer, chlorin-e6 (Ce6), thereby combining sonodynamic therapy (SDT) with DOX–MSNPs. The prepared MSNPs (using CTAB as a surfactant and TEOS as a template) were optimized for DOX delivery via initial –NH
2 and subsequent –COOH functionalization, which was observed in −31–20, 17–37 and −37 to −43 mV ranges o f
ζ-potentials for native, –NH
2 and –COOH-functionalized states, respectively. The variations suggested progressive -OH and –NH
2 replacements, with a higher colloidal stability of –COOH-functionalized MSNPs. The PS of NCs using TEM was screened as 149.5 ± 12.2 nm, with the magnitudes reaching up to 300 nm in DLS measurements, due to conjugated water molecules. The DOX release was monitored without pH fixation, reaching an 80% extent after 10 h, due to the obstruction of the MSNPs’ pores. The release was slow but not sustained due to the NCs’ surface lacking mesopores. Uptake studies deciphered a therapeutic significance of MSN–DOX–Ce6 particles, as these were internalized in MDA-MB-231 cells to a higher extent (greater fluorescence) than DOX and Ce6 alone. The release mechanism was ascertained as “free-diffusion”, with the cells requiring co-culturing with drugs for 4 h before the sonication-energized antitumor efficacy of Ce6. Screening the antitumor efficacy of MSNP–DOX–Ce6, the carriers without DOX caused no change in cell viability, while MSNP–DOX–Ce6 with ultrasound (US), decreased it more than US–Ce6, DOX+Ce6+US or free DOX. The effects were reproduced in vivo (BALB/c nude mice) with substantial TW and TV decrements for the MSNP–DOX–Ce6–US group rather than Ce6–US or DOX alone [
195]. Thereby, this study established an MSNP–DOX–Ce6 and US synergism for DOX efficacy in MDA-MB-231 TNBC treatment.
In perhaps the last major attempt from 2020, Moodley and Singh used MSNPs neat, in a CTS-functionalized state and with (2–5)% PEG functionalization for DOX and 5-FU co-delivery to MCF-7 BC cells. The as-synthesized MSNPs (CTAB as a template, TEOS as a silica precursor) were functionalized with CTS and PEG. The optimum functionalization was attained by the reversal of the anionic surface sensitivity (for MSNPs) to the cationic regime in the CTS and PEG-functionalized state. The TEM-ascertained PS for MSNPs ranged between 37 and 66 nm, while the 12–215 nm hydrodynamic diameters were due to the enhanced aqueous interactions of the silanol groups. The 710.36 m
2·g
−1 SA and 1.74 cm
3·g
−1 PV implied a nanoscale sensitivity of the prepared NPs, prevailing as graduated mesoporous spheres for drug loading and release. Encapsulation of 5-FU and DOX into the PEG-CTS@MSNPs determined a combined drug loading of 20–30%, due to their differential interactions with the carrier constituents. With a higher hydrophobicity, DOX interacted with hydrophilic PEG and was internalized to a greater extent than 5-FU, which is attracted to CTS and –NH
2 groups and is internalized afterwards. The release kinetics were monitored at pH 7.4 and 4.2, with the former resulting in a higher 5-FU + DOX liberation of 21% and 52% for NCs functionalized with 2% PEG. At pH 4.2, the release extents were 29 and 59% for 2 and 5% PEG-functionalized NCs and for 5-FU, while for DOX, these were 62 and 53%. The distinctive release extents of 5-FU and DOX were attributed to the pH-dependent 5-FU protonation, wherein a 4.2 pH caused Fickian diffusion for both drugs from 2% PEG-functionalized NCs with negligible erosion. For 5% PEG-functionalized NCs, the diffusion and erosion constants played a complementary role to modulate DOX release. Slower drug diffusion was reasoned by investigators due to DOX interaction with the MSNPs matrix and aqueous medium, besides the pH and PEG coating on the MSNPs. Screening the antitumor efficacy, the MCF-7 BC cells treated with 5-FU + DOX revealed maximum apoptosis with 2% PEG-functionalized NCs, causing DNA condensation and fragmentation. A cell cycle analysis revealed enhanced populations in S and G2/M phases, inducing DNA damage and cell cycle arrest, correlating with most of the cells undergoing apoptosis and fragmentation. These were witnessed in AO/EB-stained cells, hinting at the toxicity emanated by 5-FU and DOX-co-loaded MSNPs [
196].