1. Introduction
Drug failure occurs in vivo due to poor absorption and/or distribution, high toxicity, low solubility, and unpredicted bioavailability, as well as poor drug metabolism [
51]. Controlled and localized drug release plays a critical role in overcoming such drawbacks utilizing an appropriate drug carrier system. Lipid-based components are considered as promising materials for such delivery systems [
52,
53,
54,
55,
56].
Physicochemical properties of lipids such as biocompatibility, low susceptibility to erosion phenomena, and slow water uptake make lipids an ideal nanocarrier system to improve active pharmaceutical ingredient (API) aqueous solubility, bioavailability, and effective therapy. In addition, lipid-based systems improve API storage and delivery while inhibiting API oxidation, degradation, and decomposition [
57]. Unlike other delivery systems, lipid-based drug delivery systems exhibit a major advantage over other methods due to their ability to cross the gut, gastro-intestinal tract (GIT), blood vessels, and blood brain barrier [
58,
59]. Some lipid-based drug delivery systems include self-emulsifying systems [
60,
61], SLNs [
62,
63], nanostructured lipid carriers (NLCs) [
64,
65], and liposomes [
66].
2. Liposomes
Liposomes are regarded as the most widely applied technology for drug delivery on the market. This drug delivery system is called a lipid bilayer or a phospholipid vesicle. Liposomes have attracted a lot of attention due to their easy preparation methods and high drug loading efficiency. Further, liposomes have slow and targeted release of payloads [
67]. The term liposome defines a mesomorphic structure made of lipid, phospholipid, and water. Liposomes mainly contain phospholipid molecules, which entrap and release lipid-soluble, amphiphilic materials, as well as water-soluble compounds, in a controlled manner to enhance the efficacy of nutraceuticals, pharmaceuticals, and other bioactive compounds [
68]. Liposomes have been used in the pharmaceutical industry since 1995 thanks to US Food and Drug Administration (FDA) approval [
69].
Generally, liposomes include closed phospholipid double layers and benefit from a hydrophilic core unlike micelles. Liposomes may carry amphiphilic, hydrophobic, and hydrophilic compounds [
70,
71,
72,
73]. In other words, the polar substance is loaded into the hydrophilic core, while the hydrophobic compounds may be placed inside the bilayers of lipid domains [
73,
74]. The term nanoliposome is derived from ‘liposome’ (lipos: fat and soma: body), meaning lipidic structures with nanoscale dimensions. Nanoliposome exclusively indicates lipid vesicles at nanoscale, while liposome is considered as a general term utilized to cover different groups of lipid vesicles on the diameters ranging between tens of nanometers to several micrometers [
75].
Liposomes and nanoliposomes are categorized based on diameter range, number of internalized vesicles, and number of lamellas. Lipid vesicles may be prepared as a small unilammelar vesicles (SUVs) which are created by a bilayer membrane. Large unilammelar vesicles (LUVs) with approximately 20–100 nm diameter range benefit from a bilayer membrane, while double bilayer vesicles (DBVs) contain two bilayer membranes with above 300 nm diameter range. Oligolammelar vesicles (OLVs) contain 3–5 bilayers, while multilammelar vesicles (MLVs) contain more than five concentric bilayer vesicles. There are giant unilammelar vesicles (GUVs) which are generated by a bilayer, as well. Multivesicular vesicles (MVVs) include a bilayer liposome which encapsulates numerous small non-concentric vesicles. Nanoliposomes or sub-micron lipid vesicles are found as SUV, LUV, DBV, and OLV, while MVV, GUV, MLV, OLV, DBV, and LUV are regarded as liposomes [
75,
76]. There are numerous methods to create liposomes including reverse-phase evaporation (RPE) [
77], thin film dispersion (TFD) [
78], spray-freeze-drying [
79], and alcohol injection [
80,
81]. More environmentally friendly methods involve pro-liposomal formulation applying a cryoprotectant and high-temperature procedure, as well as supercritical fluid of carbon dioxide (SCF-CO2) techniques to adjust the experimental pressure and temperature as a method to control the shape and size of the particles [
47].
2.1. Flavonoid Liposomes
According to Jing et al., folic-acid-modified liposomes improved quercetin anti-tumor properties significantly. With this aim, a novel method of treating osteosarcoma was displayed by proving that quercetin inhibits the JAK2-STAT3-PD-L1 signaling axis role in the immune response to osteosarcoma [
82]. In addition, Tang et al. [
83] evaluated the impact of quercetin liposomes (Q-PEGL) on rats with streptozotocin (STZ)-induced diabetic nephropathy (DN) and used the technique for intragastric injection drug administration. The results indicated that quercetin-loaded liposomes could be successfully generated from proper formulation of quercetin, lecithin, cholesterol, and polyethylene glycol 4000. An animal study revealed that the formulated quercetin-loaded liposomes can demonstrate kidney-protective properties in rats with STZ-induced DN, reduce DN progression rate, and decrease AGE expression. Quercetin liposomes impact DN more than quercetin alone and can be utilized as an effective treatment for DN [
83]. A stable quercetin liposomal formulation with anti-inflammatory and antioxidant activities was prepared and studied by Ferreira-Silva et al. for treating hepatic ischemia and reperfusion injury (IRI). The results indicated that implementing quercetin in liposomal nano carriers increased therapeutic impact both in vitro and in vivo, supporting the possibility of such approach for hepatic IRI treatment [
84]. In another study, Li et al. [
85] encapsulated quercetin in the PEGylated liposomes’ non-aqueous interior and indicated that quercetin-loaded PEGylated liposomes (PEG-Que-NLs) exhibit promising anticancer effects in vitro and in vivo due to the improved solubility and bioavailability of natural quercetin. Based on the results, PEG-Que-NLs may target tumors, release medication gradually, and increase quercetin solubility. Therefore, PEG-Que-NLs may be applied in treating malignant tumors [
85]. Further, Zhang et al. [
86] proposed employing the quercetin liposome as a potential allergy antagonist to elucidate the anti-allergic action of quercetin liposomes on RBL-2H3 cells in vitro. The results indicated that quercetin liposomes can reduce the release of histamine and beta hexosaminidase, calcium influx, and expression of inflammatory markers significantly compared to quercetin alone [
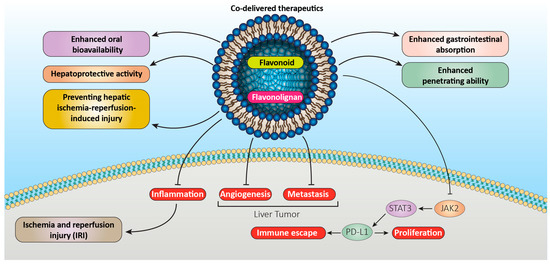
86]. Furthermore, Li et al. examined whether liposomal quercetin (LQ) may improve the outcomes of microwave ablation (MVA) while treating the rabbit VX2 liver tumor model. The results indicated that the preparative infusion of LQ can significantly improve the effects of MWA on VX2 liver tumors in rabbits, extend their survival time by inhibiting the expression of HSP70 and HIF-1 in the residual tumor, and prevent the excessive growth of the residual tumor by lowering metastasis (
Figure 2) [
87].
Figure 2. Liposomal formulations of flavonoids and flavonolignans improve their therapeutic efficacy.
Renault-Mahieux et al. proposed the idea of co-encapsulating fisetin and cisplatin into liposomes to combine the antiangiogenic properties of fisetin with the cytotoxic properties of cisplatin. Based on the results, the fisetin antiangiogenic properties remained intact, encapsulated cisplatin and fisetin affected glioblastoma (GBM) cells, and fisetin impacted GBM U87-MG cells. In other words, the co-loaded formulation exhibited effective anti-GBM cell activity and could keep fisetin effects long-lasting [
88]. In addition, Altamimi et al. reviewed the efficacy of transdermal elastic liposomes (LEL1-LEL12) loaded with luteolin (LUT) to prevent breast cancer in vitro and ex vivo. The drug-loaded carrier (OLEL1) displayed concentration-dependent inhibition of MCF-7 cells compared to the free drug and the elastic liposome improved cellular internalization for maximum inhibition, resulting in establishing a potential strategy of elastic liposomes for improving LUT transdermal administration and increasing therapeutic effectiveness in breast cancer treatment [
89]. Further, Deshmukh et al. created chrysin liposomes (CLPs) using the chitosan/lecithin/electrostatic deposition assisted film hydration approach to protect chrysin in the nano-lipoidal shell. A more than five-fold increase in chrysin liposome bioavailability was observed in in vivo pharmacokinetic research. In silico molecular docking studies of the same combination reported electrostatic interaction between the two polymers, indicating that chitosan might shield and enclose chrysin, resulting in increasing its cytotoxicity and bioavailability in the process [
90]. Furthermore, Huang et al. created a liposomal chrysin (LC) and discussed its effects on hepatic ischemia-reperfusion (HIR) and potential mechanisms. The results indicated that LC benefits from promising biocompatibility and is considered as an effective medication for treating and preventing HIR-induced injury [
91]. In another study, Tian et al. prepared PSMA-specific antibodies co-encapsulated in liposomes with genistein and plumbagin to target prostate cancer cells. The above-mentioned liposomes reduced the development of PSMA-expressing prostate cancer cells by more than 90% without harming healthy cells or human red blood cells. Overall, encapsulating other treatments utilizing the same method can create non-toxic and PSMA-specific antibody linked liposomes such as the drugs genistein and plumbagin to improve various cancer treatments and stop their spread [
92].
2.2. Flavonolignan Liposomes
In fact, one of the first SLM liposomal formulations was reported during the early 2000s. A number of factors including the drug-to-lipid ratio were discussed for preparing the aforementioned formulations [
50]. The formulation cholesterol ratio and inclusion of dicetylphosphate (DCP) as a charge inducer were optimized by injecting ethanol. A ratio of 10:2:2:1 for L-α-phosphatidylcholine (PC), SLM, cholesterol (CHOL), and DCP was applied to achieve 95% drug entrapment. The SLM-loaded liposomes benefited from a medium diameter of 390 nm and a size range of 56–1270 nm, which are regarded as appropriate for intravenous delivery during hepatoprotective tests in mice.
Ochi et al. described a recent development in the use of ligand-functionalized liposomes to increase SLM bioavailability [
93]. With this aim, a synergic effect on HepG2 cells was established by co-entrapment of glycyrrhizic acid (GA) and SLM into PEGylated liposomes. Liposomes prepared by the TFD method were formulated employing GA and SLM at a molar ratio of 1:1.74 combined with mPEG2000-DSPE, CHOL, and dipalmitoylphosphatidylcholine (DPPC) at a constant molar ratio. The results indicated that the co-encapsulated liposomes had an average diameter of about 43 nm and a zeta potential of −23.25 mV, which prevents liposome aggregation. The cellular uptake and antiviral activity of SLM encapsulated in phytoliposomes were analyzed in vitro against Huh-7.5 cells [
94]. The results indicated a 2.4-fold cell absorption compared to free SLM, as well as 300-fold more potent pharmacological activity.
Methods using the SCF-CO2 technique were utilized to create liposomes through SLM inside. In particular, liposomes applying sodium glycocholate (SGC) and soya hydrogenated L-α-phosphatidylcholine (HSPC) as the basis of lipid materials were prepared employing Solution-Enhanced Dispersion Supercritical (SEDS) fluids [
95]. The optimized products displayed improved anti-inflammatory action after comparing the liposomes created by the optimized products to those generated with more traditional techniques such as RPE and TFD. According to an in vitro analysis of the release profiles, the updated formulation enhanced SLM solubility. Based on in vivo tests, oral bioavailability of the drug improved compared to the commercial product or aqueous drug suspension. Based on the studies, bile salt stabilized vesicles (bilosomes) and SLM contents affect the drug entrapment efficiency (EE) of bilosomes created by the TFD method. Comparing the achieved liposome dispersions to the equivalents created using CHOL rather than bile salts indicated that such dispersions were classified utilizing negatively charged values. The assessed bilosomes containing sodium cholate (SC) exhibited the largest nanoparticles (NPs) in terms of particle size (595.1–98.48 nm) [
96].
Lecithin-pluronic organogels generated a novel nanocarrier for SLM in treating atopic dermatitis (AD) due to their adaptability and biphasic composition as transdermal and daily defense [
97]. The evaluated formulations contained 80% aqueous phase (pluronic) and 20% oil phase isopropyl myristate (IPM/lecithin). The symptoms and signs of patients with AD improved significantly due to the organogel hydration effect and high penetration ability.
3. Micro- and Nanoemulsions
Micro- and nanoemulsions (NEs) have been extensively studied for their ability to encapsulate a wide range of lipophilic drugs. Although the terminology may differ, both microemulsion (ME) and NE oil droplets can have sizes in the nanometer range and their size distributions may overlap. However, there is no universally accepted definition of the nanometric range, as various authors have defined critical size values with upper limits of 100 nm, 200 nm, or 500 nm [
99]. MEs contain water, oil, and surfactants which are thermodynamically stable. The small size of the droplets and surfactants contributes to the high stability and solubility of MEs, which makes them ideal for delivering drugs with low solubility. Although generally the same ingredients (oil, water, and surfactant) are required to produce NEs and MEs, the ratios of NEs and MEs may vary [
100]. MEs require a higher surfactant to oil ratio (SOR) than NEs. MEs and NEs are considered as stable and unstable, respectively, in terms of thermodynamic stability [
99]. The formation and stability of formulations is strongly influenced by the electrical properties of the surfactants. The surfactants can be classified into nonionic, zwitterionic, and cationic or anionic based on their electrical charge [
101,
102,
103].
3.1. Flavonoid Nanoemulsions
According to Shadab et al., a naringenin-loaded NE could be a potential therapeutic agent for Alzheimer disease. The direct neurotoxic effects of beta amyloid (Aβ) were reduced by a naringenin-loaded NE on SH-SY5Y cells, which was accompanied by a downregulation of amyloid precursor protein (APP), β-secretase expression. Such observations suggested ample reduction in amyloidogenesis, as well as decreased phosphorylated tau levels in human neuroblastoma cell line (SH-SY5Y) cells [
104].
Kaplan (2019) created daidzein (DZ)-containing NEs and NE-based gels (NEGs) to test their cytotoxic potential while employed topically to treat melanoma. ProtasanTM UP G 213 (1%
w/w) was added to the NE formulation after being created using a high-pressure homogenization process to generate NEG formulations. NE and NEG formulations with a controlled release profile and nanoscale droplet size were demonstrated for the topical use of DZ to treat melanoma [
105]. In addition, Hussein et al. reduced cardiac toxicity and DNA damage by administering quercetin nanoemulsion (Que-NE) to an experimental diabetes model. Based on the results, turning QCT into a NE increased its solubility. The nano-delivery method shielded diabetic rats from cardiac toxicity and DNA damage through injecting QCT which is regarded as an anti-inflammatory and antioxidant drug [
106]. Further, Mahadev et al. examined Que-NE with ultrasonically assisted dispersion for improving bioavailability and therapeutic efficacy against diabetes mellitus among rats. A Que-NE was created utilizing EO, T20, and labrasol as the appropriate oils, surfactants, and cosurfactants. The ultrasonically assisted Que-increased NE oral bioavailability raised quercetin therapeutic and preventive anti-diabetic properties significantly [
107]. Furthermore, Ceramella et al. investigated the simultaneous encapsulation of quercetin and cisplatin to simplify drug administration. Two human cell models, normal HEK-293 kidney cells and human breast carcinoma MDA-MB-231, were applied to characterize and test the derived formulations. Employing NEs containing the above-mentioned substances lessened the principal cytotoxic activity of cisplatin on HEK-293 cells significantly. In addition, the antioxidant properties of encapsulated quercetin were enhanced [
108].
In another study, Son et al. (2018) reviewed the hypocholesterolemic effects of a nanosized quercetin emulsion among rats fed with a high-cholesterol diet and characterized the physicochemical features of a double-layer oil-in-water NE encapsulated with quercetin (NQ) over a broad range of pH and various storage times. Complexation and self-assembly with Captex
® 355, Tween 80, sodium alginate, and soy lecithin were used to create a quercetin-loaded double-layer oil-in-water NE. NQ exhibited more promising outcomes than quercetin alone in decreasing blood and hepatic cholesterol levels. In addition, NQ enhanced the release of bile acid into stool among rats fed a high-cholesterol diet. Genes involved in bile acid synthesis and cholesterol efflux such as ATP-binding cassette transporter A1 (ABCA1), liver X receptor alpha (LXR), cholesterol 7 alpha-hydroxylase (CYP7A1), and ATP-binding cassette sub-family G member 1 were among those whose expression was studied by NQ (ABCG1) [
109].
Ahmadi Oskooei created quercetin nanoemulsions (QuNEs) to improve quercetin solubility in polar aqueous environments and discussed the creation of quercetin–proteins (QuNE–human serum albumin (HSA) and QuNE–holo-transferrin (HTF)). QuNE exhibited substantial antioxidant benefits due to its ability to transport quercetin to HSA and HTF proteins and stabilize their protein complexes. The synthesis of stable and bio-accessible QuNE–proteins such as QuNE–human serum albumin (HSA) and QuNE–holo-transferrin (HTF) utilizing QuNE as an appropriate primary transporter led to a secure secondary delivery system with the potential to be exploited as an effective anticancer drug [
110].
In addition, Marques analyzed the effects of quercetin and its NE on MDR and non-MDR cells applying high-pressure homogenization to make NEs. The effects of quercetin and vincristine equaled verapamil which is considered as an ABCB1 inhibitor. Docking argued that the aforementioned compounds bind to ABCB1 at a similar location. Such NE enhanced the bioavailability of quercetin by maintaining its cytotoxic and cytostatic properties. The NE ability to immediately stop ABCB1 efflux activity indicated the existence of a structure–activity link [
111].
Further, Magura (2022) assessed hesperidin NE encapsulation and compared the cytotoxicity of the optimized hesperidin-loaded NEs (HPNEM) to hesperidin alone in MCF-7. With this aim, the effect of HPNEM on MCF-7 cell cycle arrest, apoptosis, and the expression of oncomiRs (miR-155 and miR-21) were evaluated. The expression of miR-155 was downregulated by HPNEM, supporting its therapeutic potential in treating breast cancer [
112].
In another study, Colombo et al. (2018) created a muco-adhesive NE containing kaempferol (KPF-MNE) and examined its potential as a nasal delivery method for the rat brain after nasal injection, as well as its efficacy against a glioma cell line. Based on the results, the nasal mucosa was not harmed by the generated NEs, indicating that the nanocomposite film NE was regarded as superior to KPF-NE and free KPF in targeting the brain following intranasal delivery. The above-mentioned result was substantiated by ex vivo permeation experiments and in vivo bio-distribution investigations. Overall, the muco-adhesive NE increased apoptosis and reduced the viability of glioma cells [
113].
3.2. Flavonolignan Nanoemulsions
NEs and MEs with generally regarded as safe (GRAS) and food-acceptable components were developed for topical and oral administration to improve drug permeability, stability, and solubility [
114,
115,
116,
117,
118,
119].
Supersaturatable self-emulsifying drug delivery system (S-SEDDS) is designed to lessen the side effects of high surfactant levels commonly employed in SEDDS. Wei et al. [
120] led a research group to develop the aforementioned formulation with plans to improve SLM oral bioavailability. The above-mentioned formulation contained a decreased concentration of surfactant combined with hydroxypropyl methylcellulose (HPMC), which was mixed with the liquid SEDDS to induce a super saturation state through preventing or minimizing SLM precipitation. In addition, the researchers investigated the solubility of SLM in several surfactants, cosurfactants, and oils at 25 °C. Labrafac
® CC was selected as the oil phase for S-SEDDS production due to its maximum drug solubility. Cremophor
® RH 40 was selected as the surfactant due to its good emulsion-forming capabilities and the reduced size of the droplets of the optimized SLM-loaded emulsion (almost 50 nm). Cremophor
® RH 40 was selected due to the synergistic effects of the cosurfactants Labrasol
® and Transcutol
®.
4. Solid Lipid Nanoparticles
SLNs are considered as a major nanotechnology drug delivery platform [
124,
125]. SLNs are regarded as spherical particles with 50–1000 nm diameter, which contain a lipid matrix where the drug may be incorporated or dissolved [
126]. In fact, one or more room-temperature solid lipids, surfactant, and water are involved in preparing SLNs [
127]. Triglycerides, cholesterol butyrate, cholesterol, carnauba wax, beeswax, emulsifying wax, and cetyl alcohol are among the solid lipids utilized to prepare SLNs. The mechanism for lipid vesicle formation includes hydrophobic–hydrophilic interactions and the van der Waals forces of water molecules and phospholipids [
68].
SLN properties including average size, drug loading capacity, release profiles, and surface charge (i.e., zeta potential) may be precisely adjusted via selecting different structures or blends of lipids, preparation method, surfactant agent(s), and aqueous or organic production media. Different targeting moieties may be applied to functionalize the above-mentioned NPs surfaces [
128].
Nowadays, lipid-based carriers have reached high metabolic and physicochemical stability, along with higher biocompatibility [
55,
74,
129,
130]. Both hydrophilic and lipophilic drugs can be encapsulated in SLNPs with enhanced bioavailability and absorption, resulting in improving the biopharmaceutical profile compared to conventional colloidal carriers [
131]. Emulsomes are considered as the solid-state equivalents of common multi-lamellar and unilamellar lipid vesicles in which the NPs with an internal solid fat core are surrounded by at least one phospholipid layer, resulting in making emulsomes a special type of SLN [
132,
133].
4.1. Flavonoid SLNs
Rishitha assessed the effects of PTZ-induced cognitive impairment on a zebrafish model (SLN-Q) employing SLNs containing quercetin. Quercetin was found to ameliorate metabolic alterations with attenuating effect on cognition deficits which was brought on by PTZ. SLN-Q may be regarded as a potential drug in the future for treating neurodegenerative illnesses such as memory problems due to its possible anti-oxidative, anti-lipid peroxidative, and acetylcholinesterase inhibitory activities [
134].
In addition, Vijayakumar conducted the physicochemical characterization and quercetin release from various SLNs, as well as creating plain and surface-coated SLNs containing quercetin. With this aim, the effect of surface coating on quercetin cellular uptake was evaluated using Caco-2 cells. Quercetin-containing SLN formulations improved the in vitro releasing profile significantly and indicated promising stability for up to 90 days of storage at 40 °C. The SLN’s surface coating was completed successfully with no compatibility issues. SLN and c-SLN displayed better quercetin uptake into Caco-2 cells than unformulated quercetin [
135].
The effects of quercetin–solid lipid nanoparticles (QSLNs) on bone loss by ovariectomy were demonstrated in vivo in a different investigation. Quercetin and QSLNs suppressed the development of osteoclast cells and the expression of osteoclast-specific genes in in vitro examinations utilizing bone marrow cells treated with RANKL and M-CSF. Based on the study, oral administration of QSLNs at a dose of 5 mg/kg/day avoids endometrial hyperplastic outcomes and the loss of bone mass, bone strength, and microarchitecture brought on by estrogen deficiency. QSLNs showed substantial superior ability to stop bone loss [
136].
Quercetin-enriched SLNs were created applying Arabic gum as a stabilizer and stearic acid as a core lipid employing a coacervation approach [
137]. This study focuses on the synthesis and assessment of quercetin-loaded SLNs generated via coacervation. Using the aforementioned method facilitates creating SLNs containing high dose of quercetin. Based on the results, SLNs can be utilized to administer nutraceuticals like quercetin and other lipophilic medications under controlled conditions [
137].
Further, Azizi et al. studied the feasibility of combining a solid lipid (palmitic acid) and an antioxidant (quercetin) into a whey-protein-isolate-stabilized SLN emulsion to encapsule fish oil. The study was conducted to find a formulation with strong lipid and antioxidant components which entrap fish oil and provide the highest physicochemical stability. The results indicated that the physical stability of the emulsions was strengthened by adding palmitic acid and reducing the size of the oil-in-water droplets. Based on the results, quercetin boosted the oxidation stability of fish oil at low amounts of palmitic acid based on thiobarbituric acid assay [
138].
A nanoparticulate delivery system was created to protect (-)-epigallocatechin gallate (EGCG), as the green tea main bioactive component, from deterioration during storage and digestion under simulated gastrointestinal pH conditions. EGCG-loaded SLNs were created using heat homogenization (EGCG-SLNs). Pure cocoa butter was considered as the sole lipid utilized in the production process. Mono- and diglycerides (MDG) and sodium stearoyl-2-lactylate (SSL) were combined as a surfactant. The food-grade SLNs successfully safeguarded the encapsulated EGCG throughout storage under harsh conditions and neutral pH levels, resulting in adding EGCG to food products on an industrial scale [
142].
4.2. Flavonolignan SLNs
Cengiz et al. [
143] reported that SLM encapsulated in SLNs exhibited anti-hepatotoxic properties. With this aim, the formulations were applied to animals with induced hepatic damage with the co-administration of TNF-
α and the hepatotoxin D-GaIN. The intended SLNs were prepared employing a combination of Tween 80 and SLM, and hot homogenization. The zeta potential and particle size of the SLM-loaded dispersions equaled −26.5 mV and 165–200 nm, respectively. The SLM-loaded SLNs were regarded as superior to the control while treating liver damage in both in vivo and in vitro studies. In addition, Iqbal et al. [
52] extracted the organic solvent using ESE. With this aim, an organic phase containing a solid (Geleol
®)/liquid (Sefsol
® 218), as an ethanolic lipid solution, and SLM was heated at 60 °C and sonicated to emulsify at 70 °C. Then, the NLC dispersions were placed into SLMs and were enhanced utilizing the Central Composite Rotatable Design (CCRD). In the next step, the optimum formulation with a particle diameter close to 126 nm and an EE of about 85% was freeze-dried and added to the Carpol hydrogel matrix to deposit epidermal tissue suited to SLM as a chemoprevention method for skin cancer. Finally, NLCs and SEDDS were applied to treat obesity-related NAFLD and enhance SLM oral bioavailability at the GIT level [
52].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15071944