Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biology
Chronic subdural hematomas (CSDHs) are commonly encountered in elderly individuals aged over 70 years, as they tend to be associated with brain atrophy. As the population ages worldwide, the incidence of CSDH is on the rise at an estimated rate of 1.7 to 21 per 100,000 people per year.
- chronic subdural hematoma
- DBC layer
- injury
1. Introduction
Anatomically, of the three strata of the meninges, the layer at the junction of the dura and arachnoid membranes, commonly referred to as the subdural space, is composed of the lamina of the dural border cell (DBC) layer [1]. The layer is most commonly dissected by traumatic mechanisms to constitute the subdural space, which is not very compact in its architecture. In the context of brain atrophy, CSDH may arise from either acute traumatic hematoma or spontaneous hemorrhage due to disease factors. Consequently, CSDH develops when a chronic clot collects in the subdural space for approximately three months. Moreover, disruption of the subarachnoid barrier layer architecture by external forces leads to the accumulation of cerebrospinal fluid (CSF) in the subdural space and the formation of subdural hygroma. As such, the subdural space, the so-called area of structural incompetence, is created by all three of these modalities, followed by neovascularization as CSDH develops. Repeated bleeding into the subdural space eventually triggers neurological symptoms.
This type of old liquefied clot slowly and progressively accumulates in the subdural space in the supratentorial or submeningeal areas, affecting either the eloquent or non-eloquent areas of the brain or developing motor and cognitive deficits associated with basal ganglia lesions and brainstem compression symptoms. Disease expression patterns of CSDH tend to be implicit, vague, and unclear, and it is often difficult to target a specific neurological disease at first sight [2][3][4][5][6].
In general, CSDH occurs more often with subtle and non-specific manifestations such as headaches, minor alterations in mental status, and gait disturbances. Mental status changes may be characterized by dementia, unresponsiveness, or even a slightly blurred consciousness. The local neurological symptoms of CSDH manifest as cranial nerve palsy, hemiparesis, and unilateral sensory deficits, even mimicking spinal cord lesions such as flaccid paraplegia and quadriplegia. CSDH involving a supratentorial blood clot or posterior fossa may cause the following signs of brainstem compression: constricted or dilated pupil, nystagmus, limb flaccidity, eye deviation, and even a decreased level of consciousness. Furthermore, manifestations of CSDH may include seizures that generate electrophysiological abnormalities through their effects on the cerebral cortex.
2. Inflammatory Response Provoked by DBC Layer Damage as an Initiator of CSDH Development
In the following states, acute SDH due to head trauma, arachnoid rupture associated with trauma/brain surgery, hemorrhage due to physical bleeding tendency, or any stress that renders the brain atrophic, for example, aging, dehydration, or excessive drainage of CSF, the dural border cells may be torn [7][8][9], thus creating a subdural space that does not exist in the natural state.
As precursor cells for the development of fibroblastic connective tissue, when dural border cells are injured or further activated by residual blood clots in the post-acute phase, the remaining cells from the DBC layer, which have been torn and isolated either on the arachnoid surface or on the dura mater, may trigger a series of inflammatory reactions in the subdural space [10].
Once the inflammatory response is initiated, cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) are secreted in large quantities in the subdural space by stimulated connective tissue cells such as fibroblasts and immune cells [11][12]. In parallel, these inflammatory cells including neutrophils, lymphocytes, macrophages, and eosinophils can be recruited to the site, further contributing to the secretion of chemokines and eventually expanding the extent of this inflammatory cascade [13].
3. Inappropriate Inflammation Promotes the Production of Neomembranes
The aforementioned inflammatory response, as a protective mechanism in humans, aims to repair tissue damage. Inflammatory reactivity arising from the rupture of the DBC layer is followed by a further repair process that is equivalent to that of wound healing. Under the general repair of damaged tissues, collagen is deposited into the damaged or even mutilated tissue structure after injury via fibrosis. Scar tissue made up of collagen eventually fills in and replaces the original tissue remnants [14][15].
Under the pathological mechanism of CSDH, however, such a reaction is improper and excessive for the repair of dural border cells, and instead promotes inappropriate proliferation of these cells. Instead of repairing the pre-existing DBC layer, the inflammatory response proceeds to fibrosis at this disrupted interface. This process consists of the proliferation of fibrous connective tissue, which involves a high level of collagen composition and collagen matrix formation, culminating in the formation of new membranes comprising the outer and inner membranes, in approximately one week and three weeks, respectively [16]. This research team found precursors constituting core extracellular matrix (ECM) proteins such as procollagen peptides and glycosaminoglycans, at high levels within the subdural fluid of patients with head injury [17], suggesting a trend toward continuously increased synthesis of ECM in the meninges following trauma to the meninges after head trauma, that is, after cleavage of the DBC layer, as previously described.
4. Inflammation-Driven Angiogenesis Leads to Microhemorrhages on the Neomembrane Surrounding CSDH
With the aim of reconditioning cleaved DBC, the inflammatory cells present in the neomembrane, especially the outer membrane, attempt to repair the tissue with an inflammatory response. Angiogenesis is particularly relevant in this context, with a large number of new blood vessels available for increased metabolic demands of recruited infiltrating cells, tissue healing, and remodeling [18][19].
Notably, these new capillaries on the CSDH membrane are either thin or lack a basement membrane and are devoid of smooth muscle cells and pericytes, leaving the wall thin and in a highly permeable state [20]. This characteristic predisposes to recurrent microhemorrhages, along with cell transport, for example, erythrocytes, leukocytes, and plasma from the vessels into the subdural hematoma cavity [21][22], leading to hematoma enlargement.
During angiogenesis in CSDHs, vascular endothelial growth factor (VEGF), derived from neutrophils, macrophages in the fluid, or vascular endothelial cells in the CSDH membrane, initiates the activation of endothelial cells in the vessels, allowing for cell proliferation, cell migration, and subsequent formation of a vascular lumen [23][24]. Furthermore, matrix metallopeptidase-9 (MMP-9), a protein hydrolase that contributes to angiogenesis, has been found to increase vascular permeability and enhance the inflammatory response [25], which may contribute to CSDH enlargement. In combination with VEGF, MMPs may impair neovascularization stability, potentially contributing to a higher bleeding risk [22].
Moreover, an angiographic study identified that neovascularization was not limited to the outer membrane but was also found in the inner membrane of the hematoma [20][26]. Neovascularization from the inner membrane may be associated with CSDH recurrence after embolization of the middle meningeal artery (EMMA), an alternative treatment [27].
5. Hyperfibrinolysis Causes CSDH to Be Less Susceptible to Coagulation, Creating a Vicious cycle along with Microbleeding from Neovascularization
CSDH, a highly variable hematoma pattern, originates from the detachment of the DBC layer, as described previously. The subsequent series of pathological reactions, subtly appear as a process of wound healing. After repeated micro-hemorrhages from neovascularization, an initial hemostatic effect is implemented as a result of normal physiological function, followed by a sequence of inflammation, remodeling, and fibrosis, where the originally formed fibrin bridge is further removed in preparation for tissue granulation [28]. As a result, coagulation and fibrinolysis occur repeatedly in CSDH, and under such a vicious cycle, a sustained hyperfibrinolytic state eventually emerges, which provides a plausible explanation for the tendency of hematoma enlargement and recurrence after surgery.
It is worth pondering that the predominant immune cells underlying the pathogenesis of CSDH are eosinophils, and not microglia, the major innate immune cells of the CNS. Eosinophils can promote mobility throughout the CSDH by releasing plasminogen to induce excessive fibrinolysis [16]. However, like many inflammatory substances with a double-edged nature, they have a corresponding role in promoting the maturation of hematomas [29].
The above-mentioned crucial pathological mechanisms with regard to the generation of CSDH, corresponding to the wound healing process, are demonstrated in Figure 1.
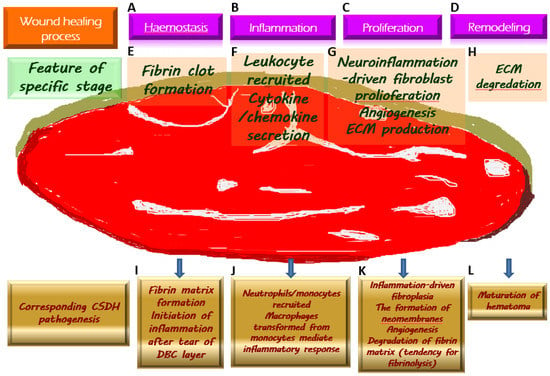
Figure 1. Chronic subdural hematoma (CSDH) depicted as an intracranial wound tending to not heal properly. Once a wound is created, the physiologically protective mechanism undergoes hemostasis (A) and the formation of fibrin clot (E), which serves as an extracellular matrix (ECM) for the migration of immune cells recruited by pro-inflammatory cytokines (F) along the subsequent inflammatory response (B). During the proliferative phase (C), fibroblasts undergo proliferation while producing ECM and generating neovascularization to provide for the nutritional needs of the cells (G). When the wound is about to heal, the ECM is gradually degraded by the remodeling process (D) and is replaced by scar tissue (H). Regarding the formation of CSDH, the tear of the dural border cell (DBC) layer subtly corresponds to the creation of a wound. Fibrin matrix formation provides a comprehensive explanation for the presence of coagulation signals in the effusion of CSDH (I). Subsequently, under the vicious cascade of inflammatory response (J), fibroblast proliferation, and ECM generation contribute to neomembrane production. Angiogenesis takes place in the emerging inner and outer membranes, and the newly formed vessels have fragile walls, resulting in repeated microbleeding. In addition, during this proliferative phase, the tissue that resembles granulation replaces the originally generated fibrin matrix, thereby leading to a tendency of fibrinolysis (K). With the maturation of the inner and outer membranes in CSDH, the neovascularization is less likely to rupture and bleed, indicating the coming wound remodeling and eventual stabilization of CSDH (L).
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13020235
References
- Derk, J.; Jones, H.E.; Como, C.; Pawlikowski, B.; Siegenthaler, J.A. Living on the Edge of the CNS: Meninges Cell Diversity in Health and Disease. Front. Cell. Neurosci. 2021, 15, 703944.
- Herath, H.; Matthias, A.T.; Kulatunga, A. Acute on chronic bilateral subdural hematoma presenting with acute complete flaccid paraplegia and urinary retention mimicking an acute spinal cord injury: A case report. BMC Res. Notes 2017, 10, 627.
- Kumar, A.S.; Alugolu, R. Chronic subdural hematoma presenting as diplegia-A rare presentation. J. Neurosci. Rural Pract. 2014, 5, 445–446.
- Monserrate, A.; De Jesus, O. Huge chronic subdural haematoma formation in an encephalomalacia stroke cavity. BMJ Case Rep. 2020, 13, e236928.
- Won, S.Y.; Dubinski, D.; Sautter, L.; Hattingen, E.; Seifert, V.; Rosenow, F.; Freiman, T.; Strzelczyk, A.; Konczalla, J. Seizure and status epilepticus in chronic subdural hematoma. Acta Neurol. Scand. 2019, 140, 194–203.
- Sundblom, J.; Sandberg, E.; Ronne-Engström, E. Trauma Mechanisms and Surgical Outcomes in the Elderly Patient with Chronic Subdural Hematoma. Can. Geriatr. J. 2022, 25, 40–48.
- Miki, K.; Abe, H.; Morishita, T.; Hayashi, S.; Yagi, K.; Arima, H.; Inoue, T. Double-crescent sign as a predictor of chronic subdural hematoma recurrence following burr-hole surgery. J. Neurosurg. 2019, 131, 1905–1911.
- Kim, B.O.; Kim, J.Y.; Whang, K.; Cho, S.M.; Oh, J.W.; Koo, Y.M.; Hu, C.; Pyen, J.S.; Choi, J.W. The Risk Factors of Subdural Hygroma after Decompressive Craniectomy. Korean J. Neurotrauma 2018, 14, 93–98.
- Lee, K.S. Chronic Subdural Hematoma in the Aged, Trauma or Degeneration? J. Korean Neurosurg. Soc. 2016, 59, 1–5.
- Dorrier, C.E.; Jones, H.E.; Pintarić, L.; Siegenthaler, J.A.; Daneman, R. Emerging roles for CNS fibroblasts in health, injury and disease. Nat. Rev. Neurosci. 2022, 23, 23–34.
- Nguyen, H.N.; Noss, E.H.; Mizoguchi, F.; Huppertz, C.; Wei, K.S.; Watts, G.F.M.; Brenner, M.B. Autocrine Loop Involving IL-6 Family Member LIF, LIF Receptor, and STAT4 Drives Sustained Fibroblast Production of Inflammatory Mediators. Immunity 2017, 46, 220–232.
- Osuka, K.; Watanabe, Y.; Usuda, N.; Iwami, K.; Miyachi, S.; Takayasu, M. Expression of high mobility group B1 and toll-like receptor-nuclear factor κB signaling pathway in chronic subdural hematomas. PLoS ONE 2020, 15, e0233643.
- Osuka, K.; Ohmichi, Y.; Ohmichi, M.; Nakura, T.; Iwami, K.; Watanabe, Y.; Miyachi, S. Sequential Expression of Chemokines in Chronic Subdural Hematoma Fluids after Trepanation Surgery. J. Neurotrauma 2021, 38, 1979–1987.
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol. Aspects Med. 2019, 65, 2–15.
- Zhang, M.; Zhang, S. T Cells in Fibrosis and Fibrotic Diseases. Front. Immunol. 2020, 11, 1142.
- Holl, D.C.; Volovici, V.; Dirven, C.M.F.; Peul, W.C.; van Kooten, F.; Jellema, K.; van der Gaag, N.A.; Miah, I.P.; Kho, K.H.; den Hertog, H.M.; et al. Pathophysiology and Nonsurgical Treatment of Chronic Subdural Hematoma: From Past to Present to Future. World Neurosurg. 2018, 116, 402–411.e2.
- Heula, A.L.; Sajanti, J.; Majamaa, K. Glycosaminoglycans in subdural fluid and CSF after meningeal injury. Acta Neurochir. 2015, 157, 2105–2110; discussion 2110.
- Barchitta, M.; Maugeri, A.; Favara, G.; Magnano San Lio, R.; Evola, G.; Agodi, A.; Basile, G. Nutrition and Wound Healing: An Overview Focusing on the Beneficial Effects of Curcumin. Int. J. Mol. Sci. 2019, 20, 1119.
- Wang, C.; Zhang, Z.; Xu, T.; Lou, Y.; Wang, Q.; Jin, H.; Zhang, L.; Feng, Y.; Xu, H.; Mao, C. Upregulating mTOR/ERK signaling with leonurine for promoting angiogenesis and tissue regeneration in a full-thickness cutaneous wound model. Food Funct. 2018, 9, 2374–2385.
- Moshayedi, P.; Liebeskind, D.S. Middle Meningeal Artery Embolization in Chronic Subdural Hematoma: Implications of Pathophysiology in Trial Design. Front. Neurol. 2020, 11, 923.
- Nouri, A.; Gondar, R.; Schaller, K.; Meling, T. Chronic Subdural Hematoma (cSDH): A review of the current state of the art. Brain Spine 2021, 1, 100300.
- Edlmann, E.; Giorgi-Coll, S.; Whitfield, P.C.; Carpenter, K.L.H.; Hutchinson, P.J. Pathophysiology of chronic subdural haematoma: Inflammation, angiogenesis and implications for pharmacotherapy. J. Neuroinflamm. 2017, 14, 108.
- Han, J.M.; Gou, M.; Xiao, R. Neutrophils regulate the process of angiogenesis. Sheng Li Xue Bao 2017, 69, 843–851.
- Michaeli, S.; Dakwar, V.; Weidenfeld, K.; Granski, O.; Gilon, O.; Schif-Zuck, S.; Mamchur, A.; Shams, I.; Barkan, D. Soluble Mediators Produced by Pro-Resolving Macrophages Inhibit Angiogenesis. Front. Immunol. 2018, 9, 768.
- Su, G.J.; Gao, J.; Wu, C.W.; Zou, J.F.; Zhang, D.; Zhu, D.L.; Liu, J.; Zhang, J.H.; Huang, X.J. Serum Levels of MMP-8 and MMP-9 as Markers in Chronic Subdural Hematoma. J. Clin. Med. 2022, 11, 902.
- Saito, H.; Tanaka, M.; Hadeishi, H. Angiogenesis in the Septum and Inner Membrane of Refractory Chronic Subdural Hematomas: Consideration of Findings after Middle Meningeal Artery Embolization with Low-concentration n-butyl-2-cyanoacrylate. NMC Case Rep. J. 2019, 6, 105–110.
- Dian, J.; Linton, J.; Shankar, J.J. Risk of recurrence of subdural hematoma after EMMA vs surgical drainage—Systematic review and meta-analysis. Interv. Neuroradiol. 2021, 27, 577–583.
- Bayer, I.S. Advances in Fibrin-Based Materials in Wound Repair: A Review. Molecules 2022, 27, 4504.
- Davidson, B.; Narvacan, K.; Munoz, D.G.; Rotondo, F.; Kovacs, K.; Zhang, S.; Cusimano, M.D. The Crucial Role of Eosinophils in the Life Cycle, Radiographical Architecture, and Risk of Recurrence of Chronic Subdural Hematomas. Neurotrauma Rep. 2021, 2, 76–83.
This entry is offline, you can click here to edit this entry!
