Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
Motor symptoms dominate the clinical expression of PD. Muscular rigidity, akinesia, bradykinesia, gait instability, and resting tremor form the core of the motor symptoms. The concept of “parkinsonism” encompasses all motor impairments. For the clinical diagnosis, parkinsonism is defined as bradykinesia accompanied by resting tremor, rigidity, or both. Dopamine (DA) loss secondary to degeneration of the substantia nigra pars compacta (SNc) initiates parkinsonism by causing impaired modulatory function in the motor network.
- network
- neurophysiology
- oscillation
1. Introduction
Neurodegenerative diseases were one of the first ailments to receive medical attention worldwide [1]. Clinically, these diseases show heterogeneous and overlaying symptoms. Hence, a framework that hones diagnosis will allow better specific treatment and management. Parkinson’s disease (PD) is one of the leading neurodegenerative disorders with heightened prevalence [2,3]. It is considered a movement disorder, although it is accepted that the classic motor symptoms are accompanied by a myriad of nonmotor symptoms, as well as hyposmia, urinary dysfunction, orthostatic hypotension, memory loss, depression, pain, gastrointestinal dysfunction, and sleep disturbances [4]. Gastrointestinal symptoms include drooling, dysphagia, disabled gastric emptying, constipation, and impaired defecation [5,6].
Several cellular mechanisms, including mitochondrial dysfunction, oxidative stress, neuroinflammation, and deficient protein degradation are implicated in the pathogenesis of PD. Nonetheless, the pathological fingerprint consists of neural inclusions of Lewy bodies (LBs) and Lewy neurites, with cell loss in the substantia nigra and other brain areas. The burgeoning of LBs begins from an initial template of alpha-synuclein, which incites the seeding of nearby alpha-synuclein proteins, which triggers the formation of aggregates into a toxic, insoluble pleated sheet structure, to form LBs [7]. At the network neurophysiology level, these pathological fingerprints lead to rearrangement in the electrophysiological and neurophysiological activity that generates the symptoms [8,9,10].
Despite the above, the diagnosis is complicated by the overlap of motor and nonmotor symptoms, in addition to the possibility of other neurodegenerative diseases; as a result, disease management remains suboptimal [4]. In recent years, the criteria for diagnosis have been designed and validated and are dependent on the presence of motor symptoms [4,11,12].
2. Network Involvement in Motor Symptoms
Motor symptoms dominate the clinical expression of PD. Muscular rigidity, akinesia, bradykinesia, gait instability, and resting tremor form the core of the motor symptoms [9,11,12]. The concept of “parkinsonism” encompasses all motor impairments. For the clinical diagnosis, parkinsonism is defined as bradykinesia accompanied by resting tremor, rigidity, or both [7,11,12]. Dopamine (DA) loss secondary to degeneration of the substantia nigra pars compacta (SNc) initiates parkinsonism by causing impaired modulatory function in the motor network [7,9,13].
Motor output is modulated by the basal ganglia (BG). The BG comprises several nuclei: the striatum (Str), the external (GPe) and internal (GPi) segments of the globus pallidus (GP), the subthalamic nucleus (STN), the substantia nigra compacta (SNc) and the reticulata (SNr). Functionally, the cortex (Cx) sends motor information by excitatory axons to the Str, STN, and thalamus (Th). In this way, the information reaches the circuit through the Str and emerges through the output nuclei, the GPi/SNr, which then send the information to Th.The Str dually organizes the circuits according to the projection neurons that send their axons to the output nuclei. Thus, the connection between the Str and GPi/SNr forms the “direct” pathway. At the same time, the Str establishes a connection, before reaching the output nuclei, with the GPe and the STN, giving rise to the “indirect” pathway (Str-GPe-STN-GPi/SNr; Figure 1B left) [14,15,16]. In turn, this network forms larger parallel circuits that include the frontal Cx, ventral Th, and two nuclei of the brainstem: the superior colliculus [17] and the pedunculopontine nucleus (PPN) [18]. Based on the functions of the cortical area of origin, the BG-Th-Cx network is designated as “motor”, “associative/cognitive”, and “limbic” circuits [8,19]. In our context, parkinsonism arises from abnormal activity patterns in the motor circuit (Figure 1C left).
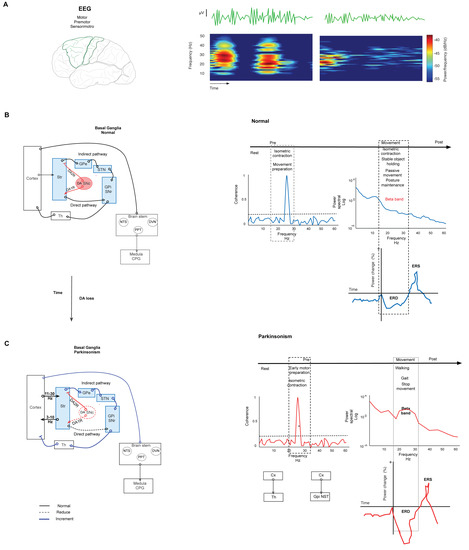
Figure 1. Basal ganglia network in normal and parkinsonism conditions. (A) Spectrogram representation of EEG recording of power in beta-band activity in motor and premotor cortex. The spectrogram shows changes in power at the frequency of the beta band. (B) Left. Simplified schematic of the BG network in normal conditions. The BG modulate the motor output through the projection neurons of the Str that send their axons to the output nuclei: GPi/SNr in two pathways. The direct path connects the Str with GPi/SNr. In the indirect path, the Str connects with the GPe, the STN, and GPi/SNr. Synthesized by SNc, DA is the network modulator through two types of receptors expressed in both pathways. D1 receptors are expressed in the direct path, and D2 receptors are expressed in the indirect path. Under normal conditions, DA release in the Str reduces the combination of these effects, modulating the GPi and SNr activity, and thus it reduces the inhibition of thalamocortical neurons. Right. Graph representation of changes in the parameters of EEG in normal conditions in several stages of movement. The coherence increments are during isometric contraction and training preparation. Beta power declines during movement performance, postural maintenance, and stable object holding. The same movement events show the desynchronization of beta power (ERD). (C) Simplified schematic representation of the BG network in parkinsonism. Left. Degeneration of the nigrostriatal pathway causes DAloss-induced aberrant transmission of the sensorimotor Str and reduces both the direct pathway’s tonic excitation and the indirect pathway’s tonic inhibition; consequently, the BG overinhibit their thalamic and brainstem targets. This causes motor symptoms and aberrant oscillation in beta-band frequency. Right. Changes in beta-band frequency are shown in coherence, power, and ERD during motor performance. The coherence increment is during isometric contraction and movement preparation between CxM-Th and CxM-Gpi/NST. The power density of an ERD shows increment during gait, walking, and movement cessation. Continuous lines represent physiological connections. The dashed line represents a decrease. The bold line represents increased activity. SNc. Substantia nigra compacta. GPi. Globus pallidus internus. GPe. Globus pallidus external. STN. Subthalamic nucleus. Str. Striatum. The graft was elaborated based on the data reported in the main text and supported by the respective references.
Synthesized in the SNc, DA is a critical modulator in the network. Expressed in both direct and indirect pathways, DA receptors are coupled to different second messenger systems (through Gs or Golf for D1-like receptors and Gi or Go for D2-like receptors). In the direct pathway, DA acts on D1 receptors to inhibit the BG output nucleus. DA acts on D2 receptors in the indirect pathway to suppress activity. Under normal conditions, DA release in the Str reduces the combination of these effects under GPi and SNr activity, reducing the inhibition of thalamocortical neurons that receive the input from the output nucleus (Figure 1B) [20,21]. Thus, the loss of DA by nigrostriatal pathway degeneration induces aberrant transmission of the sensorimotor striatum [21] (more strongly than transmission to the associative and limbic regions); consequently, this pathway degeneration allows GPe-STN activity to go into overdrive, thus raising the inhibition of STN neurons and their projections to output nuclei [22,23]. This change causes parkinsonism (Figure 1C left).
Neuronal activity patterns play an essential role in determining the integrative functions of the BG. In neural ensembles, information is transmitted through temporal patterns of action potentials [24]. Therefore, it is accepted that the information is encoded in the firing rate of individual neurons [25]. In this context, changes in the firing rate of individual neurons in some specific nuclei of the BG induced by DA loss explain the pathophysiology of parkinsonism. In the network, the loss of DA reduces the direct pathway’s tonic excitation and the indirect pathway’s tonic inhibition [13,21]. Both changes increase the mean firing rates of output nuclei. Consequently, the BG overinhibits their thalamic and brainstem targets [26]. This causes decreased activity in Th and Cx, resulting in akinesia.
Secondary to the loss of DA, the Str and the Th show changes in their firing rate. In the Str, the neurons projecting to the direct pathway show decreased spontaneous activity, while those of the indirect pathway show increased spontaneous activity [8]. In the Th, neurons show a slowed firing rate [27], and their firing is modulated during reaching movements [8]. The GPe shows a decreased firing rate after DA depletion [28], and at the same time, the local levels of GABA are increased [29,30]. Reports of changes in the motor cortex (CxM) in parkinsonism are scarce. Recently, the decreased firing of neurons projecting to the pyramidal tract was shown, but did not affect those projecting to the Str [31]. These results suggest that transmission from cortical neurons to pyramidal tract neurons might be involved in motor symptoms associated with parkinsonism [8]. It has been proposed that cortical firing activity could be secondary to dysfunction in the BG and Th and possible changes in the Cx secondary to the loss of DA and other neurotransmitters [32]. However, other studies show findings related to the alterations associated with parkinsonism, both in firing frequency and patterns and network synchrony.
Firing Pattern Implication in the Neurophysiology of the BG Network
Physiologically, the action potential is the canonical form of information transmission in the brain. Nevertheless, it is known that specific neurons, based on their intrinsic electrical properties, can show increased mean firing frequency over a short time; this type of activity is described as the burst pattern [13,33].
In the BG normal network, the firing pattern of the GPi, GPe, and STN neurons is random, although action potentials do not occur in bursts. In this condition, the GPi fires action potentials continuously at high frequency. In the same way, the GPe fires action potentials at high frequency, although with pauses, and the STN also fires action potentials continuously but in a medium range of frequencies [13]. These characteristics change considerably secondary to DA depletion. Extensive evidence allows us to accept that DA depletion modifies the intrinsic properties of the neurons of different nuclei of the circuit [34,35,36,37]. Similarly, the GPe shows an increase in the range in which burst firing occurs, adding to the diminished firing rate [38,39]. Similarly, the STN shows a modified firing pattern similar to bursting and increased firing frequency [40]. Notably, burst activity in the STN has been resolutely correlated with clinically severe parkinsonism in patients with PD [41].
The reciprocal connection between the GPe and STN inside the network is physiologically transcendental. Both cores are considered the pacemaker of the network [42], and this notion is given particular importance in the development of bursts [23]. Higher activity from the indirect pathway onto the GPe that guides rhythmicity is demonstrated after DA loss by increased density in the synaptic link between both cores; consequently, the GPe increases inhibition that causes a hyperpolarization-induced higher burst pattern in STN neurons [23,42]. In addition, during parkinsonism, neurons of the GPe and GPi increase synchronization, and the GPi tends to fire in a burst pattern [43].
Thalamic neurons have intrinsic properties that allow them to burst under normal conditions or exhibit tonic bursting depending on the physiological state [44]; the burst pattern trends for the BG nuclei are similar to those in the Th [45], especially in the motor region [40,45]. Notably, burst activity results from a convergence of axons from the cerebellum in the Th motor regions [8]. In this sense, a connection between the cerebellum and BG was described recently and suggested implications for parkinsonism symptoms [8,46]. Therefore, the cerebellum-Th-Cx network contributes to parkinsonism [47].
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13142394
This entry is offline, you can click here to edit this entry!
