Nanobiosensors are sensors in which various nanosize materials are used, however, large equipment is still required for whole sensing systems, because they are not, by themselves, able to detect nanoscale events. The current state-of-the-art nanobiosensors are based on several diagnostic methods, such as enzyme-linked immunosorbent assay (ELISA), flow cytometric immunoassay, electrochemical (amperometric, voltammetric and impedance), immunoassay, mass spectrometric immunoassay, chromatographic immunoassay, and different optical immunoassays. Contemporary multiplexed nanobiosensors are developed for the detection and quantification of clinically relevant analytes in biological and clinical samples (i.e., blood, urine, and saliva).
- multiplexing
- nanoparticles
- quantum dots
- gold nanoparticles
- silver nanoparticles
- upconverting nanoparticles
1. Introduction
The clinical diagnosis of many diseases depends on the accurate and unambiguous detection of various biomarkers, which may include proteins or other biomolecules [1,2,3]. Immunoassays are a well-established tool for measuring analytes that are normally present at very low concentrations and cannot be determined accurately by other less expensive tests. Standard immunoassays are used to detect one specific analyte, however, for complex diseases such as cancer, diabetes, rheumatoid arthritis (RA) and osteoarthritis (OA), which involve a multitude of biomarkers, one analyte is not enough to obtain an early and accurate diagnosis. In this case, multiple immunoassays can be employed. However, immunoassays require samples that are collected, labelled, archived, and bio-banked according to established laboratory protocols before even conducting the assays, which makes this method challenging and time-consuming. In order to diagnose at the earliest stages of disease development, it is crucial to detect as much information as possible from small quantities of clinical samples. In this context, multiplexed immunoassays are an obvious and attractive approach, especially when sample volumes are limited. The main advantage of multiplexed analysis is the capability to detect multiple biomarkers qualitatively or quantitatively in a single sample. There are additional advantages to this approach, such as more data points from single samples, reduced cost per data point, fewer errors due to fewer samples, and increased throughput (Figure 1). Conversely, cross-reactivity remains a significant challenge. If antibodies cannot distinguish between the analyte and other structurally similar components, the specificity of immunoreactivity drastically decreases, and as a consequence, multiplexed detection may not work properly in complex biological and clinical samples.
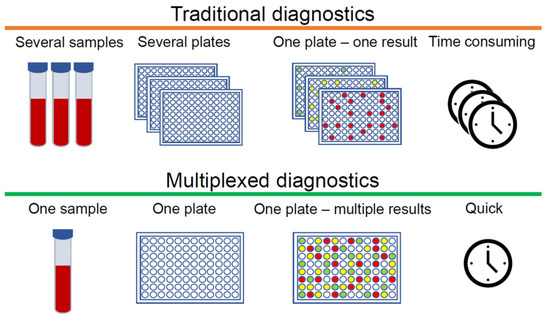
Figure 1. Differences between traditional and multiplexed diagnostics.
2. Gold and Silver Nanoparticles
One of most widely used groups of nanoparticles are synthesised from noble metals, such as gold and silver. Gold (Au) and silver (Ag) nanoparticles (AuNPs and AgNPs) are the most stable metal nanoparticles, and they have numerous attractive features such as size-related electronic, magnetic and optical properties (quantum size effect), large optical field enhancements resulting in the strong scattering and absorption of light, and their perspective applications in biomedical field [51]. Noble metal nanoparticles, which are composed of tens or hundreds of atoms and typically < 2 nm size, are known as nanoclusters. These nanoclusters have molecule-like properties due to their extraordinary physical and chemical characteristics, and they have especially attracted the attention of scientists [52]. AuNPs are known as non-toxic and biocompatible nanoparticles; thus they are frequently used in studies related to diagnostic tool development, drug delivery, novel therapeutic agents, and other medical applications [53,54]. Whereas, AgNPs have unique antimicrobial features, which allows silver nanoparticles to be used in the treatment of microorganisms such as bacteria, fungi, and viruses [55]. In this article, the application of AuNPs and AgNPs for multiplexed biomarkers detection will be briefly reviewed.
2.1. Plasmonic Multiplex Sensing
Gold and silver nanoparticles are exceptional due to their plasmonic properties. Such nanoparticles demonstrate unique absorption and scattering characteristics in the VIS–NIR spectral region due to photon-induced collective oscillation of their surface electrons. This coherent electron oscillation in noble metal nanoparticles is also known as localised surface plasmon resonance (LSPR). Briefly, LSPR occurs when a nanoparticle (bigger than 2 nm) is interacting with light. Electromagnetic oscillating fields cause the conduction electrons of a nanoparticle surface to oscillate coherently. A highly localised oscillating electron cloud is created around the nanoparticle, which rapidly decays away, through resonance enhance far-field scattering. The LSPR profile can be described with spectroscopically measured parameters such as shape, position and intensity, which strongly depend on the properties of nanoparticles, such as size, shape, monodispersity, as well as interaction with ligands or surrounding media [56].
Huang et al. (2012) developed a multiplexed bioanalytical assay which allows simultaneous detection of human serum specimens infected by Schistosoma japonicum and tuberculosis pathogens without sample pre-treatment. Two different populations of gold nanorods (AuNRs) were modified with antibodies of different antigens. Detection of biomarkers was achieved by registering a shift in the LSPR, using a standard visible/NIR spectrometer. This nanobiosensor was able to identify infected and uninfected samples and provide a semi-quantitative readout [57].
Utilising the LSPR properties of plasmonic metal nanoparticles, a new variation of the conventional ELISA was established, known as plasmonic ELISA (pELISA) [10]. Several studies have demonstrated the application of pELISA for diagnostics of diseases such as prostate cancer [58,59,60], syphilis [61] tuberculosis [62], hepatitis B [63] and HIV [64]. However, all pELISA systems are designed for one analyte, and despite its great potential there are no multiplexed pELISAs thus far.
2.2. Multiplexed Colorimetric Detection
The nanoparticle-based colorimetric assays are one of the most attractive methods for the detection of various biomolecules such as DNA, RNA, enzymes, proteins, and other small molecules. The key parameter for colorimetric sensing is the ability to change colour of colloidal solution due to changes of noble metal nanoparticles size or distance between them. Such colorimetric sensors can be divided into four types: aggregation, etching, growth and nanoenzyme [65]. The most commonly used gold or silver nanoparticle-based sensors are of the aggregation type, in which optical features of solutions change depending on their level of aggregation. During aggregation of nanoparticles, the surface plasmons of the particles couple and their LSPR profile changes. The main advantages of colorimetric assays are that results can be monitored with the naked eye without the need for any instrumentation. Additionally, this method is simple, convenient, and low cost. Most studies have developed colorimetric detection tools for one analyte [66], however, some multiplexed colorimetric assays have been published.
Mancuso et al. used AuNPs and AgNPs for Kaposi’s sarcoma associated herpesvirus and Bartonella DNA simultaneous detection [67]. Specific DNA primers, which recognise targeted oligonucleotide sequences, were attached to AuNPs and AgNPs. When targeted oligonucleotides appear in solution, nanoparticles conjugated with primers bond with them and with each other. Consequently, aggregates of AuNPs and AgNPs are formed and the solution changes colour. Changes were identified visually and by measuring the absorption of the solutions. Authors have demonstrated that the proposed method works when AuNPs and AgNPs are mixed in the same solution—both colour change reactions can be seen independently of each other. In this assay, the limit of DNA detection for the AuNPs is 2 nM and for the AgNPs is 1 nM [67].
Heo et al. demonstrated two-plexed colorimetric assay using three types of nanoparticles, functionalised with DNA: gold nanoparticles (AuNP-DNA), silver nanoparticles (AgNP-DNA), and gold nanorods (AuNR-DNA) [68]. The mixture of these nanoparticles is black-coloured, but after targeted analytes are introduced, the colour of the solution changes, based on which type of nanoparticles (or combination) have aggregated. The authors used a modified CMYK (cyan, magenta, yellow, and key (black)) colour model: when one type of nanoparticle aggregates, corresponding colours are detracted from the mixture. For example, when red-coloured AuNPs aggregate due to interaction with targeted analytes, the mixture turns light-green (cyan and yellow combination). Using this approach, the authors successfully demonstrated the detection of thrombin and platelet-derived growth factor (PDGF) in human blood plasma. The sensitivity of this method was not high (the limit of detection of PDGF was 19 nM), however, it was possible to visually detect the detection limit and concentration (20 nM) with the naked eye [68].
Different approaches for the multiplexed colorimetric diagnostic detection of cancer was demonstrated by Di et al. [69]. The authors used decorated AuNPs and antibody conjugated exosomes for a nanozyme-assisted immunosorbent assay to detect CD63, CEA, GPC-3, PD-L1 and HER2 exosomal proteins from four cell lines as well as from clinical serum samples. This method allows users to differentiate the levels of the various proteins without additional labelling with detection antibodies, enabling the development of a quicker and much simpler testing procedure [69].
2.3. Multiplex SERS Imaging
Surface-enhanced Raman scattering (SERS) is a method for precise molecular detection based on the enhanced Raman scattering of biomolecules, which are localised on nanostructured SERS-active gold or silver surfaces. The advantages of this method are very high sensitivity down to the single molecule level, and the sharp molecularly specific spectra. The use of SERS instead of QD labelling for imaging is superior because SERS provides greater sensitivity for molecular analysis [70]. Moreover, the Raman signals exhibit much narrower peaks compared with traditional fluorophores and QDs; generally SERS signals are only 1–2 nm width [71]. Additionally, Raman signals are exceptionally stable and resistant to photobleaching, independently from measurement conditions. Thus, SERS tags are absolutely applicable for the multiplex detection of soluble biomarkers as well as multiplexed SERS-based cellular imaging (Figure 5).
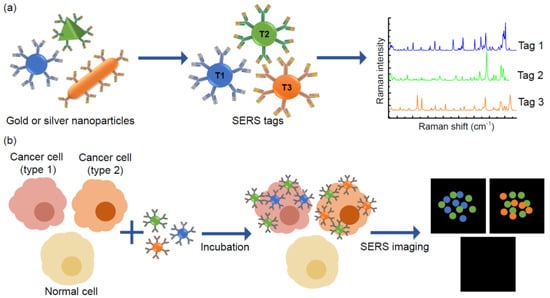
Figure 5. Principles of multiplexed detection using the surface-enhanced Raman scattering (SERS) technique. (a) Noble metal nanoparticles, that are different in size and shape, have unique Raman signals with narrow peaks, thus they could be used as SERS tags. (b) Schematic illustration of simultaneous detection of three tumour associated antigens expressed different types of cancer cells by SERS imaging.
Lee et al. (2012) demonstrated SERS-based cellular imaging technique to detect and quantify multiple breast cancer biomarkers expressed on plasma membranes [72]. Silica-encapsulated AuNPs were conjugated with CD24 and CD44 antibodies and fluorescent dyes (FITC and RuITC), creating dual mode nanoprobes (SERS and fluorescence). They demonstrated that simultaneous detection of CD24 and CD44 biomarkers in breast cancer cells (MDA-MB-231) can be enhanced by combining the two methods: fluorescence imaging for quick detection and SERS imaging for accurate localisation of biomarkers.
Later studies by the same group applied a SERS-based imaging method for the multicolour detection of three breast cancer cell biomarkers [73]. This study was caried out in human breast cancer cell lines (MDA-MB-468, KPL4 and SK-BR-3) by measuring the expression of EGF, ErbB2, andIGF-1 biomarkers. After visualisation, SERS-mapping images were recorded, which allowed them to localise and quantify EGFR, ErbB2 and IGF-1R proteins in cells. Analysis of the experimental results enabled identification and phenotyping of cancer cells [73].
Sun et al. (2015) used polydopamine encapsulated SERS probes for the detection of tumour-associated biomarkers (VEGF, EGFR and Vimentin) in different prostate cancer cell lines (LnCAP, PC-3, DU145) [74]. By using SERS images, the expression of three tumour biomarkers on the surface of prostate cancer cells were evaluated visually and three prostate cancer cell lines had been distinguished from each other. The authors suggest that usage of the multiplexed SERS imaging technique could improve the identification of cancer cell phenotypes [74].
Li et al. (2018) developed a multiplexed nanobiosensor by adapting the sandwich-type immunoassay and SERS methods for the detection of cancer biomarkers. Three soluble cancer protein biomarkers (soluble programmed death 1 (sPD-1), soluble programmed death-ligand 1 (sPD-L1) and soluble epithermal growth factor receptor (sEGFR)) were analysed directly from human serum [75]. In this study, for Raman signal improvement, gold-silver alloy nanoboxes were used as SERS tags to facilitate highly sensitive detection. The limit of detection for sPD-1, sPD-L1, and sEGFR achieved by this platform was 6.17 pg/mL, 0.68 pg/mL, and 69.86 pg/mL, respectively. They proposed that their nanobiosensor has huge potential in the clinic, because it can accurately and specifically detect several cancer biomarkers in human serum at once [75].
Another study demonstrated multiplex profiling of oestrogen receptor (ER), progesterone receptor (PR), and epidermal growth factor receptor (EGFR) expression in breast cancer tissue and normal tissue sections, using AuNP-based SERS tags [76]. 60 nm AuNPs with incorporated alkyne and nitrile groups and conjugated to primary antibodies against the growth factors were used as SERS tags. They demonstrated that their SERS nanotags have individual and definite bands in cellular regions without any Raman signal. The method was tested in vitro using the MCF-7 cell line, which expresses ER, EGFR, and PR at high levels and the normal cell line 3T3 in which expression of the biomarkers is downregulated. The results corresponded with data obtained with other methods. Additionally, the authors examined the use of multiple SERS tags for ER, EGFR, and PR imaging in human breast cancer tissue specimens. The results showed higher expression levels of these three biomarkers in breast cancer tissues compared to healthy samples, consistent with the diagnostic and prognostic classification of these tissues. Thus, the authors suggest that their proposed method has potential for multiplex imaging in clinical cancer diagnosis [76].
SERS is a reliable analytical method suitable for the detection of multiple analytes in biological samples even if there are extremely low quantities of target biomarker. By using SERS-nanotags, assays can be conducted directly in biopsy samples as well as in tissues or live cells, if necessary. However, utilisation of noble metal nanoparticles in multiplexed SERS biosensors is not yet cost effective and has limitations, such as high-cost fabrication of nanoparticles, low batch-to-batch consistency, high complexity, and relatively low specificity. Reproducible methodologies suitable for the mass production of testing kits based on gold or silver SERS nanotags also remain a challenge. Additionally, the majority of published multiplexed SERS imaging techniques require specific equipment, thus it would be complicated to adapt these methods for clinical needs. Despite these challenges, the field of multiplex SERS has great potential for innovation in diagnostic applications.
2.4. Plasmon-Enhanced Multiplexed Biosensing
Plasmon-enhanced fluorescence (PEF) is a phenomenon by which the fluorescence intensity of a nearby fluorophore can be remarkably enhanced by a plasmonic nanostructure [77]. By utilising PEF, the quantum efficiency and photostability of fluorophores are increased. Additionally, extremely low amounts of fluorophore could be detected. Thus, these features enable sensitive detection for very low abundance biomarkers. For the past few years, PEF-based biosensors have received a great deal of attention for the ultrasensitive detection of single analytes. Ventura et al. used the surface of patterned AuNPs for FITC fluorescence enhancement to detect immunoglobulins in real urine samples and showed that their method works in a 10–100 µg/L detection range with a limit of detection of 8 µg/L [78]. Zhang et al. used an Au nanohole array for prostate-specific antigen detection and demonstrated a limit of detection of 140 fM [79].
Wang et al. demonstrated thrombin and platelet-derived growth factor-BB (PDGF-BB) simultaneous detection using aptamer-modified AgNPs as a capture substrate, and fluorescent dye-modified aptamers as detection probes in a sandwich immunoassay [80]. The linear range of thrombin detection was from 55.6 pM to 13.5 nM, with the limit of 6.2 pM. PDGF-BB was detected with the range of concentration from 625 pM to 20 nM and the detection limit was 156 pM. They showed that by using AgNPs probes for Cy3 and Cy5 fluorescence enhancement, the detection limit could be improved 80-fold for thrombin and 8-fold for PDGF-BB, when compared to aptamers without enhancement [80].
Liu et al. developed a multiplexed antibody microarray for circulating biomarkers associated with lung cancer detection [81]. Microarray plates were modified with gold nanostructures to enhance the fluorescence of IRDye800, which was used to label detection antibodies in sandwich immunoassay. Lung cancer biomarkers CEA, CYFRA 21-1 and NSE were detected directly from human serum. Simultaneous detection of three biomarkers was achieved by printing capture antibodies onto 3 × 3 spot matrices. This approach enables a high-throughput testing of lung cancer biomarkers and improved specificity and sensitivity compared with convenient methods such as ELISA and Luminex assay [81]. Similar studies published by the same group demonstrated detection of diabetes biomarkers [82] as well as zika and dengue viruses [83].
Min et al. demonstrated a new and simple technique that allows the multiplexed detection of biomarkers in extracellular vesicles [84]. In this study, plasmon-enhancement was achieved by using Au nanoholes as a substrate. Firstly, vesicles were captured on Au nanoholes by a biotin-avidin reaction. Then, vesicles were stained with antibodies conjugated with fluorescent labels (Alexa Fluor 488, Cy3, Cy5, Cy5.5). Au nanoholes operated as amplifiers for fluorophores under excitation. They applied the designed assay to detect glioblastoma biomarkers CD-pan (CD9, CD63, and CD81), with EGFR, EGFRvIII and GAPDH as control markers, from supernatants of glioblastoma cells. Fluorescence signals of multiple fluorophores were amplified by one order, and both transmembrane and intravesicular biomarkers were detected at the single extracellular vesicle level. However, the proposed assay still has limited multiplexing capability, which is dependent on fluorescent microscope systems, allowing the visualisation of up to three or four fluorophores in one sample. Additionally, this system was not tested with clinical samples, thus its real applicability still is unknown [84].
Liu et al. developed an assay for the detection of SARS-CoV-2 antibodies by using nanostructured plasmonic gold substrate as a near-infrared fluorescence amplifier [85]. In this study, IgG and IgM antibodies against SARS-CoV-2 were detected directly from human serum and saliva using a sandwich immunoassay in microarray plate. They demonstrated high specificity and sensitivity for the detection of antibodies against SARS-CoV-2. Additionally, antibodies to viruses associated with common colds did not cross-react with SARS-CoV-2 or SARS, or reaction was low. Application of this fast and sensitive testing method in daily clinical practice would allow population-based diagnostic mass screening of COVID-19 [85].
Reference (we'll rearrange the references after you submitted it)
- Song, Y.; Huang, Y.-Y.; Liu, X.; Zhang, X.; Ferrari, M.; Qin, L. Point-of-care technologies for molecular diagnostics using a drop of blood. Trends Biotechnol. 2014, 32, 132–139.
- Nimse, S.B.; Sonawane, M.D.; Song, K.-S.; Kim, T. Biomarker detection technologies and future directions. Analyst 2016, 141, 740–755.
- Kosaka, N.; Kogure, A.; Yamamoto, T.; Urabe, F.; Usuba, W.; Prieto-Vila, M.; Ochiya, T. Exploiting the message from cancer: The diagnostic value of extracellular vesicles for clinical applications. Exp. Mol. Med. 2019, 51, 1–9.
- Tao, Y.; Li, M.; Ren, J.; Qu, X. Metal nanoclusters: Novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663.
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556.
- Jeong, H.-H.; Choi, E.; Ellis, E.; Lee, T.-C. Recent advances in gold nanoparticles for biomedical applications: From hybrid structures to multi-functionality. J. Mater. Chem. B 2019, 7, 3480–3496.
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354.
- Moores, A.; Goettmann, F. The plasmon band in noble metal nanoparticles: An introduction to theory and applications. New J. Chem. 2006, 30, 1121–1132.
- Huang, H.; Liu, F.; Huang, S.; Yuan, S.; Liao, B.; Yi, S.; Zeng, Y.; Chu, P.K. Sensitive and simultaneous detection of different disease markers using multiplexed gold nanorods. Anal. Chim. Acta 2012, 755, 108–114.
- Liang, J.; Yao, C.; Li, X.; Wu, Z.; Huang, C.; Fu, Q.; Lan, C.; Cao, D.; Tang, Y. Silver Nanoprism Etching-Based Plasmonic ELISA for the High Sensitive Detection of Prostate-Specific Antigen. Biosens Bioelectron 2015, 69, 128–134.
- Liu, D.; Yang, J.; Wang, H.-F.; Wang, Z.; Huang, X.; Wang, Z.; Niu, G.; Hight Walker, A.R.; Chen, X. Glucose oxidase-catalyzed growth of gold nanoparticles enables quantitative detection of attomolar cancer biomarkers. Anal. Chem. 2014, 86, 5800–5806.
- Rodríguez-Lorenzo, L.; de la Rica, R.; Álvarez-Puebla, R.A.; Liz-Marzán, L.M.; Stevens, M.M. Plasmonic nanosensors with inverse sensitivity by means of enzyme-guided crystal growth. Nat. Mater. 2012, 11, 604–607.
- Nie, X.-M.; Huang, R.; Dong, C.-X.; Tang, L.-J.; Gui, R.; Jiang, J.-H. Plasmonic ELISA for the ultrasensitive detection of Treponema pallidum. Biosens. Bioelectron. 2014, 58, 314–319.
- Mohd Bakhori, N.; Yusof, N.A.; Abdullah, J.; Wasoh, H.; Md Noor, S.S.; Ahmad Raston, N.H.; Mohammad, F. Immuno Nanosensor for the Ultrasensitive Naked Eye Detection of Tuberculosis. Sensors 2018, 18, 1932.
- Peng, M.-P.; Ma, W.; Long, Y.-T. Alcohol Dehydrogenase-Catalyzed Gold Nanoparticle Seed-Mediated Growth Allows Reliable Detection of Disease Biomarkers with the Naked Eye. Anal. Chem. 2015, 87, 5891–5896.
- Cecchin, D.; de la Rica, R.; Bain, R.E.S.; Finnis, M.W.; Stevens, M.M.; Battaglia, G. Plasmonic ELISA for the detection of gp120 at ultralow concentrations with the naked eye. Nanoscale 2014, 6, 9559–9562.
- Chang, C.-C.; Chen, C.-P.; Wu, T.-H.; Yang, C.-H.; Lin, C.-W.; Chen, C.-Y. Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications. Nanomaterials 2019, 9, 861.
- Sabela, M.; Balme, S.; Bechelany, M.; Janot, J.-M.; Bisetty, K. A Review of Gold and Silver Nanoparticle-Based Colorimetric Sensing Assays. Adv. Eng. Mater. 2017, 19, 1700270.
- Mancuso, M.; Jiang, L.; Cesarman, E.; Erickson, D. Multiplexed Colorimetric Detection of Kaposi’s Sarcoma Associated Herpesvirus and Bartonella DNA using Gold and Silver Nanoparticles. Nanoscale 2013, 5, 1678–1686.
- Heo, J.H.; Yi, G.S.; Lee, B.S.; Cho, H.H.; Lee, J.W.; Lee, J.H. A significant enhancement of color transition from an on-off type achromatic colorimetric nanosensor for highly sensitive multi-analyte detection with the naked eye. Nanoscale 2016, 8, 18341–18351.
- Di, H.; Mi, Z.; Sun, Y.; Liu, X.; Liu, X.; Li, A.; Jiang, Y.; Gao, H.; Rong, P.; Liu, D. Nanozyme-assisted sensitive profiling of exosomal proteins for rapid cancer diagnosis. Theranostics 2020, 10, 9303–9314.
- Lee, H.; Gao, X.; Kim, Y.-P. Immuno-Nanoparticles for Multiplex Protein Imaging in Cells and Tissues. BioChip J. 2018, 12, 83–92.
- Schlücker, S. Surface-enhanced Raman spectroscopy: Concepts and chemical applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795.
- Lee, S.; Chon, H.; Yoon, S.-Y.; Lee, E.K.; Chang, S.-I.; Lim, D.W.; Choo, J. Fabrication of SERS-fluorescence dual modal nanoprobes and application to multiplex cancer cell imaging. Nanoscale 2012, 4, 124–129.
- Lee, S.; Chon, H.; Lee, J.; Ko, J.; Chung, B.H.; Lim, D.W.; Choo, J. Rapid and sensitive phenotypic marker detection on breast cancer cells using surface-enhanced Raman scattering (SERS) imaging. Biosens. Bioelectron. 2014, 51, 238–243.
- Sun, C.; Zhang, L.; Zhang, R.; Gao, M.; Zhang, X. Facilely synthesized polydopamine encapsulated surface-enhanced Raman scattering (SERS) probes for multiplex tumor associated cell surface antigen detection using SERS imaging. RSC Adv. 2015, 5, 72369–72372.
- Li, J.; Wang, J.; Grewal, Y.S.; Howard, C.B.; Raftery, L.J.; Mahler, S.; Wang, Y.; Trau, M. Multiplexed SERS Detection of Soluble Cancer Protein Biomarkers with Gold–Silver Alloy Nanoboxes and Nanoyeast Single-Chain Variable Fragments. Anal. Chem. 2018, 90, 10377–10384.
- Li, M.; Wu, J.; Ma, M.; Feng, Z.; Mi, Z.; Rong, P.; Liu, D. Alkyne- and Nitrile-Anchored Gold Nanoparticles for Multiplex SERS Imaging of Biomarkers in Cancer Cells and Tissues. Nanotheranostics 2019, 3, 113–119.
- Fothergill, S.M.; Joyce, C.; Xie, F. Metal enhanced fluorescence biosensing: From ultra-violet towards second near-infrared window. Nanoscale 2018, 10, 20914–20929.
- Della Ventura, B.; Gelzo, M.; Battista, E.; Alabastri, A.; Schirato, A.; Castaldo, G.; Corso, G.; Gentile, F.; Velotta, R. Biosensor for Point-of-Care Analysis of Immunoglobulins in Urine by Metal Enhanced Fluorescence from Gold Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 3753–3762.
- Zhang, Q.; Wu, L.; Wong, T.I.; Zhang, J.; Liu, X.; Zhou, X.; Bai, P.; Liedberg, B.; Wang, Y. Surface plasmon-enhanced fluorescence on Au nanohole array for prostate-specific antigen detection. Int. J. Nanomed. 2017, 12, 2307–2314.
- Wang, Y.; Li, H.; Xu, D. Aptamers-based sandwich assay for silver-enhanced fluorescence multiplex detection. Anal. Chim. Acta 2016, 905, 149–155.
- Liu, B.; Li, Y.; Wan, H.; Wang, L.; Xu, W.; Zhu, S.; Liang, Y.; Zhang, B.; Lou, J.; Dai, H.; et al. High Performance, Multiplexed Lung Cancer Biomarker Detection on a Plasmonic Gold Chip. Adv. Funct. Mater. 2016, 26, 7994–8002.
- Zhang, B.; Kumar, R.B.; Dai, H.; Feldman, B.J. A plasmonic chip for biomarker discovery and diagnosis of type 1 diabetes. Nat. Med. 2014, 20, 948–953.
- Zhang, B.; Pinsky, B.A.; Ananta, J.S.; Zhao, S.; Arulkumar, S.; Wan, H.; Sahoo, M.K.; Abeynayake, J.; Waggoner, J.J.; Hopes, C.; et al. Diagnosis of Zika virus infection on a nanotechnology platform. Nat. Med. 2017, 23, 548–550.
- Min, J.; Son, T.; Hong, J.-S.; Cheah, P.S.; Wegemann, A.; Murlidharan, K.; Weissleder, R.; Lee, H.; Im, H. Plasmon-Enhanced Biosensing for Multiplexed Profiling of Extracellular Vesicles. Adv. Biosyst. 2020, 2000003.
- Liu, T.; Hsiung, J.; Zhao, S.; Kost, J.; Sreedhar, D.; Hanson, C.V.; Olson, K.; Keare, D.; Chang, S.T.; Bliden, K.P.; et al. Quantification of antibody avidities and accurate detection of SARS-CoV-2 antibodies in serum and saliva on plasmonic substrates. Nat. Biomed. Eng. 2020, 1–9.
This entry is adapted from the peer-reviewed paper 10.3390/s20236890
