Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Organic
Asymmetric enamine base activation of carbonyl compounds is a well-known and widely used strategy for providing functionalization of organic compounds in an efficient way. The use of solely organic substances, which in most cases are commercially available primary or secondary amines that are easy to obtain, avoids the use of hazardous substances or metal traces, making this type of catalysis a highly convenient methodology from a sustainable point of view.
- dual activation
- aminocatalysis
- asymmetric synthesis
1. Introduction
In 2000, List, Lerner and Barbas III presented the intermolecular cross aldol reaction between acetone and diversely substituted aldehydes promoted by the simple and natural amino acid (S)-proline [1] (Scheme 1a). This was not the first time that this amino acid was employed as the catalyst in an organic transformation [2,3], but it was presented as a powerful and broad-in-scope catalyst for performing intermolecular cross aldol reactions, also comparable to the best organometallic catalysts for carrying out the same reaction. In this report, based on a previous reaction mechanism of aldolases described by Barbas III [4] and confirmed by computational studies carried out by Houk and List [5], the proposed mechanism included a dual behavior of the catalyst: firstly, it formed an intermediate enamine that increased the nucleophilicity of the α-carbon in acetone, and secondly, it was also involved in the activation of the aldehyde, the second carbonyl unit (Scheme 1b). This catalyst could also provide excellent yields and enantioselectivities using a simple reaction design: stirring the aldehyde and 30 mol% of (S)-proline in a mixture of acetone/DMSO (1:4) for 2–48 h.
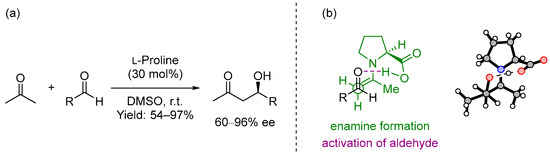
Scheme 1. (a) Proline-catalyzed asymmetric aldol reaction and (b) proposed transition state.
The use of proline as catalyst, together with the well-known MacMillan imidazolidinone introduced the same year [6,7], represented the starting point in a new research area of great interest: aminocatalysis. This type of catalyst has emerged as a valuable tool for the alleviation of some problems associated with metal catalysis related to trace contamination of those elements. In fact, aminocatalysis [8,9,10,11] and other types of organocatalysis [12,13,14,15,16,17,18] have been recognized by researchers as one of the most important strategies aligned to green and sustainable catalysis [19]. Aminocatalysis also represents the strategy of choice in many asymmetric transformations, especially when carbonyl compounds are involved, offering good results in terms of yields and enantioselectivities.
In this area, numerous examples of successful reactions have made extensive use of the incorporation of an H-bond donor entity either as an external additive or as a component of a bifunctional aminocatalyst [20,21,22,23,24,25,26]. In bifunctional H-bond donor/aminocatalysts, the H-bond donor reagent typically plays a role in the activation of an electrophile that reacts with an enamine-type intermediate. As a result, it becomes a potent stereodirecting component (Scheme 2).
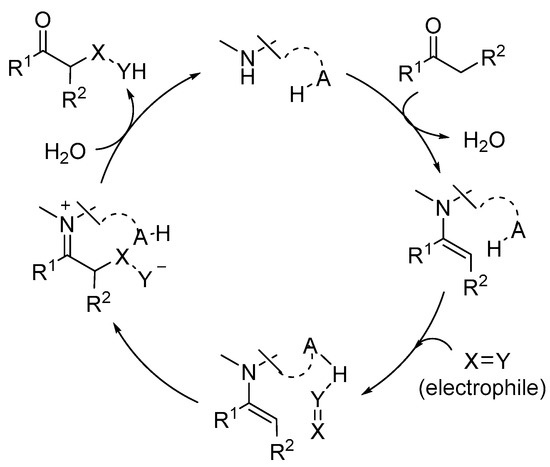
Scheme 2. Illustration of dual activation of reagents in enamine base catalysis.
Alternately, the H-bond donor moiety can also play an important role in events that occur prior to or after the stereogenic center’s installation, either as part of a bifunctional reagent or as an external cocatalyst. This occurs when a Brønsted acidic cocatalyst or stoichiometric additive is added to the reaction mixture to speed up the rate of catalyst turnover during the hydrolysis step or to make it easier to activate the carbonyl group of the aldehyde or ketone substrate during the condensation with the aminocatalyst in the activation stage. This science is beyond the scope of this paper and will not be examined exhaustively.
2. Dual H-Bonding and Enamine Activation
An excellent illustration of the involvement of an H-bond donor site in the structure of the aminocatalyst during the aldol reaction between the intermediate enamine and the external aldehyde reagent is the pioneering proline-catalyzed aldol reaction (Scheme 3). As a result, outstanding levels of both diastereo- and enantioselectivity are achieved as the reaction progresses through a cyclic chair-like transition state with reduced conformational mobility. This generated H-bond during the C-C bond forming step has been used as a general strategy during experimental and computational studies [5,27,28,29,30,31,32,33,34]. In fact, the conformational restricted transition state does not just record the extremely high facial selectivity; this situation restricts the E diastereoisomer formed in the transient enamine species, as the presence of serious dynamic and thermodynamic restrictions in the less favored Z diastereoisomer (see Scheme 2).
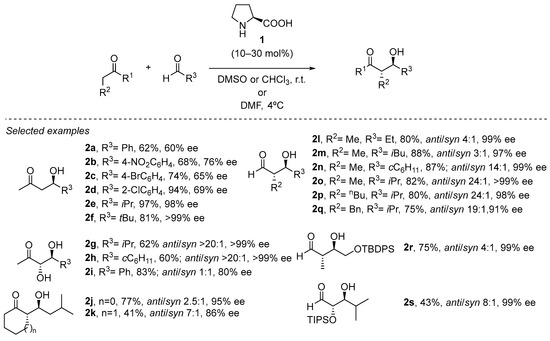
Scheme 3. General proline-catalyzed cross-aldol reaction.
This transformation is rather wide in scope with respect to the possibility of using different aldehydes in combination with acetone [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56], α-hydroxyketones [57,58] or cyclic ketones [59] as the pronucleophile source, including the possibility of performing the reaction at multigram or even kilogram scale. Moreover, the reaction can also be carried out in open air and without the need for degassed or dry solvent, which is an important benefit in terms of operational simplicity. In addition, the cross-aldol reaction between two different aldehydes can also be carried out under slightly modified conditions [60], also enabling the use of polyhydroxylated aldehydes as either pronucleophiles or electrophiles [61], which also opens the possibility for the stereoselective synthesis of sugars through this methodology [62].
The same stereochemical model can be applied to structurally related electrophiles, thus expanding the portfolio of organic transformations in which this approach can be applied [63,64]. In particular, proline has been demonstrated to be an outstanding catalyst for performing the Mannich reaction (Scheme 4) [65,66,67,68,69]. In this case, the availability of a single electron pair at the nitrogen atom available to engage in the H-bonding interaction with the carboxylate group of the proline leads to the formation of the syn diastereoisomer as a consequence of the (E) geometry of the azomethine moiety, in contrast to the anti diastereoselection previously observed in the parent aldol reaction (see Scheme 4). Also, the possibility of performing the challenging cross-Mannich reaction using acetaldehyde as the pronucleophile should be highlighted, providing acceptable yields of the corresponding α-unsubstituted β-aminoaldehydes and minimizing to a great extent the presence of byproducts arising from competitive polymerization through self-aldol condensation [70].
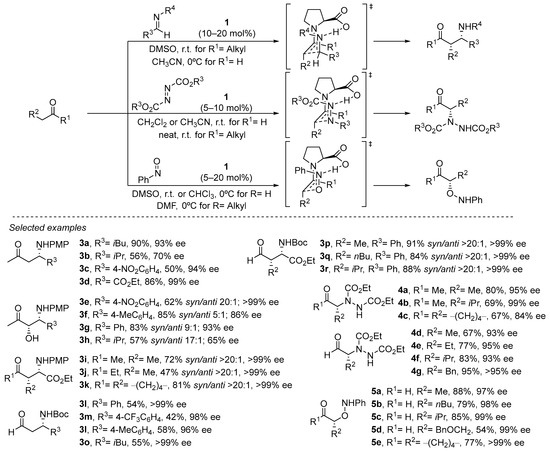
Scheme 4. Proline-catalyzed α-functionalization reactions.
Other electronically similar electrophiles such as azodicarboxylates or nitrosoarenes also perform excellently in the α-amination [71,72,73,74,75,76] and the α-hydroxylation [77,78,79,80,81,82,83] (or nitroso-aldol reaction) of aldehydes and ketones under proline catalysis, providing an efficient and direct entry to enantioenriched α-aminocarbonyl or α-hydroxycarbonyl compounds or related derivatives (See Scheme 4). In the latter case, the participation of such an H-bonding interaction with the electrophile also conditions the chemoselectivity of the reaction, observing that Jørgensen–Hayashi catalysts that do not contain any H-bond donor motif also efficiently catalyze the reaction between aldehydes and nitrosobenzene but lead to the formation of the opposite N-addition isomer, thus resulting in the α-hydroxyamination of the starting material [84].
There are still a few situations in which the reaction is limited due to either poor enantiocontrol or low conversion, despite the fact that proline performs exceptionally well in many of these reactions and has a rather broad substrate scope. This has been credited by and large to the unfortunate dissolvability of proline in the normal natural solvents utilized for the response. A possible solution has been found through the incorporation of achiral H-bond donor additives able to engage in H-bonding interactions with the carboxylate moiety that can increase the solubility of the catalyst and also contribute to the activation of the electrophile through the H-bonding interactions. This is the case of the combined use of l-proline and chiral BINOL 6, which, used in the correct matched combination and under optimized ratio, provided a remarkable improvement in the aldol reaction between acetone and several aromatic aldehydes compared to the parent reaction without any additive (Scheme 5) [38]. Further research on this behavior has led to improved systems that combine the use of l-proline with an external achiral thiourea cocatalyst [47,85,86,87] as H-bond donor.
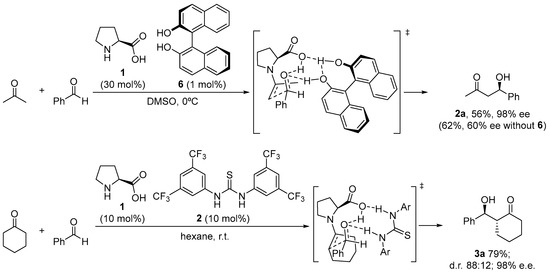
Scheme 5. Proline/H-bond additive catalyzed asymmetric aldol reaction.
Obviously, proline is not a universal catalyst for this type of transformation, and a wide variety of structurally related catalysts based on the proline scaffold have been studied and employed with different degrees of success in aldol [88], Mannich [89,90,91], α-amination [92] or α-aminoxylation [93] involving the enamine activation of aldehydes and ketones. The logical evolution of the initial system relied on the modification of the hydrogen-bond donor moiety, also tuning its acidity and availability or incorporating multiple additional H-bond donor units within its structure [94,95]. Some selected examples that provide an overall view of the different directions taken in this area are displayed in Figure 1. For instance, simply changing from the carboxylate moiety on proline to the corresponding N-arylamide 7 [96,97,98] or N-sulfonylamide 8 [99,100,101,102,103,104,105] leads to competent catalysts in such transformations. The same applies to the substitution of the carboxylate with other related motifs, as in the case of prolinamide 9 [106,107,108,109] or pyrrolidine-tetrazole catalyst 10 [110,111,112,113,114] or the possibility of using modified versions of trans-4-hydroxyproline as in the case of compound 11 [115,116]. As alternatives, several authors have also surveyed the incorporation of substituents with additional stereogenic elements like α-amino acids [117,118,119,120], sugar moieties [121] or other, more complex alkaloids [122,123,124] (see, for example, catalysts 12, 13 and 14). Another strategy has also relied on incorporating functionalities with additional H-bond donor motifs that lead to the formation of a transition state in which both reagents, the enamine intermediate and the electrophile are connected through a network of H-bonding interactions that turn into reduced conformational mobility. Some examples include the use of aminoalcohol-derived prolinamide 15 [96,125,126] or diamine-based catalysts such as 16 [127,128] or 17 [129,130,131] that also incorporate additional stereogenic elements on the N-substituent or even with a terminal thiourea moiety that provides enhanced H-bond donor ability [132,133,134,135,136] (see catalyst 18 for a representative example). Alternatively, dipeptide-type catalysts [137,138,139] with a terminal secondary amide moiety (like in catalysts 19 [140] or 20 [141]) or more complex polypeptidic scaffolds (see catalysts 21 [142] and 22 [143] as examples), which are based on either natural or unnatural [144] amino acid scaffolds, have also proved to be useful in this type of reaction (23) [145].
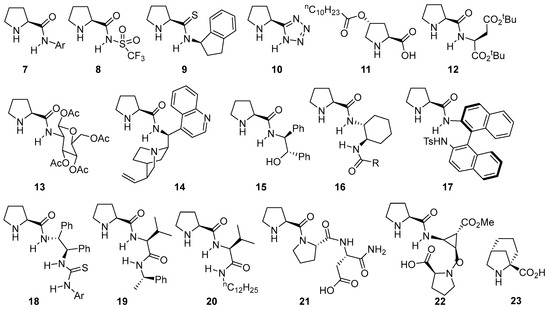
Figure 1. Selected examples of bifunctional proline-based aminocatalysts incorporating H-bond donor moieties employed in aldol, Mannich and related reactions.
Interestingly, moving the carboxylate group in the pyrrolidine core from the 2-position to the 3-position leads to a huge difference in how the electrophile and the enamine intermediate organize in the transition state. For instance, the use of pyrrolidine-3-carboxylate 24 in the Mannich reaction leads to a complete change in the simple diastereoselection of the reaction, moving from the syn-selectivity reported for the proline-catalyzed reaction (see Scheme 4) to provide the anti Mannich adducts in excellent yield and stereocontrol (Scheme 6) [146]. This behavior was explained through the formation of an H-bonded intermediate in which the chair-like TS operating in the l-proline-catalyzed reaction—in which the H-bonded imine approaches the reactive (E)-s-trans enamine conformer—was no longer operating, thus moving to a situation in which—while the carboxylate still directs the incoming electrophile from the same face of the (E)-enamine intermediate—this enamine has changed its reactive conformation to s-cis, thus exposing the opposite diastereotopic face. A similar behavior has also been described for related catalyst systems based on trans-4-hydroxyproline [147,148] or trans-3-aminoproline [149,150].
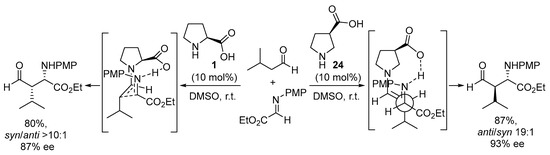
Scheme 6. The proline- vs. 3-pyrrolidinecarboxylate-catalyzed enantioselective Mannich reactions.
A similar effect in which internal H-bonding changes the reactive conformation of the enamine intermediate in order to provide opposite simple diastereoselection to those observed in the archetypical l-proline-catalyzed aldol or Mannich reactions has been explored by Maruoka and coworkers with bifunctional catalyst 25a based on the binaphthyl core (Scheme 7) [151,152,153,154,155,156]. In this case, an acidic sulfonamide substituent at the 3-position of the binaphthyl core plays the role of the stereodirecting element as an H-bond donor motif.
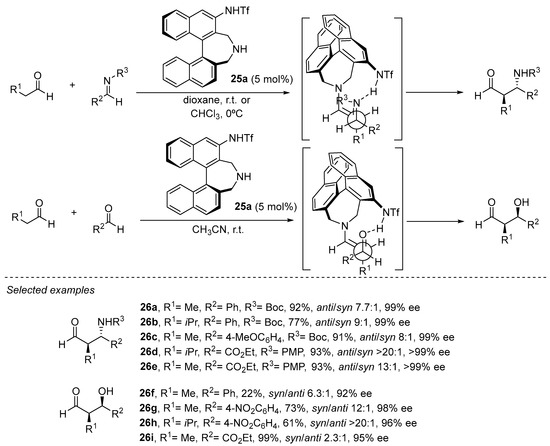
Scheme 7. Binaphthyl-based chiral catalyst 24a in enantioselective aldol and Mannich reactions.
Primary amines have been demonstrated to perform well in aldol, Mannich, α-amination and α-aminoxylation reactions [133,157,158]. The enamine formed after condensation with a primary amine has a reduced degree of steric congestion around the enamine moiety and, therefore, enables the activation of more sterically demanding substrates such as, for example, acyclic ketones or α,α-disubstituted aldehydes. In addition, the NH moiety of the secondary enamine is able to engage in H-bonding interactions with the incoming electrophile or with additional Lewis basic sites of the catalysts, facilitating the formation of a geometrically defined intermediate and, therefore, a higher degree of stereocontrol. In fact, the smallest natural chiral α-amino acid, L-alanine (27), has demonstrated its proficiency in catalyzing the aldol reaction (Scheme 8) [159], and other reports afterwards demonstrated that several other proteinogenic α-amino acids were also good catalysts for aldol and related reactions [160].
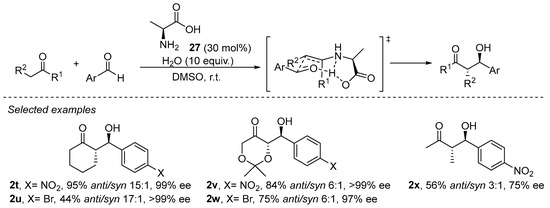
Scheme 8. The L-alanine-catalyzed enantioselective aldol reaction.
There are also several other bifunctional catalysts involving primary amines that incorporate additional H-bond donor entities reported for these transformations (see Figure 2 for several representative examples), starting with simple modifications of the a-amino acid core like, for example 28 [161]), chiral diamine-based compounds like 29 [162] or 30 [163] and also dipeptides, tripeptides or even longer peptide compounds (illustrative cases: 31 [164], 32 [165,166] and 33 [167]).
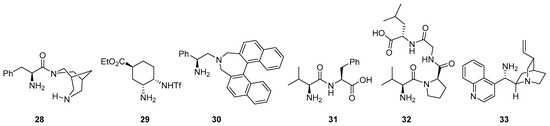
Figure 2. Selected examples of bifunctional primary amine/H-bond donor catalysts employed in aldol, Mannich and related reactions.
The use of α,β-unsaturated carbonyl compounds as electrophiles in the Michael reaction with aldehydes and ketones under enamine catalysis deserves special attention [168]. The fact that the Lewis basic site at the Michael acceptor that has to interact with the H-bond donor motif of the catalyst is placed at a longer distance in comparison with the previously discussed electrophiles entails that most of the bifunctional hydrogen bond donor/aminocatalysts that perform well in the aldol, Mannich, α-hydrazination or α-aminoxylation tend to provide poor results in the Michael reaction. For example, the l-proline-catalyzed Michael reaction between cyclohexanone and β-nitrostyrene proceeds and provides the corresponding Michael adduct in excellent yield and diastereoselectivity but with poor enantiocontrol [169], and the same applies to tetrazole analogue 10 [170], but increasing the distance between the secondary amine moiety and the H-bond donor site, as in homoproline/tetrazole catalyst 34, led to a significant improvement in the enantioselectivity (Scheme 9) [171]. In this sense, the well-known ability of thioureas to establish persistent interactions through double H-bonding events with the nitro group also has been applied to the design of efficient bifunctional pyrrolidine/thiourea catalysts such as 35 [172,173], which also performs well in the Michael reaction using nitroalkenes as electrophiles [174,175,176,177,178].
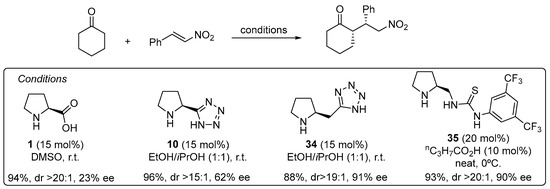
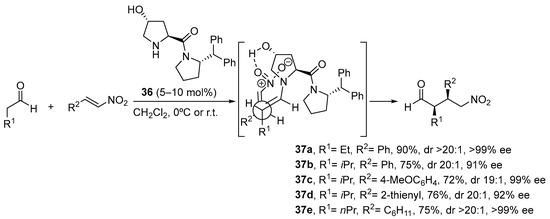

Scheme 9. The Michael reaction between cyclohexanone and β-nitrostyrene catalyzed by proline and tetrazolylpyrrolidine vs. functionalized pyrrolidines 34 and 35. As an alternative, trans-4-hydoxy-l-prolinamide 36 has also been described to promote very efficiently the enantioselective Michael reaction between aldehydes and nitroalkenes (Scheme 10) [179]. In this case, the facial selectivity is reversed with respect to the reactions catalyzed by l-proline or the related derivatives shown in the previous scheme, which results from the internal activation of the nitroalkene by the 4-OH moiety of the enamine intermediate. The bulky amide substituents also contribute to enhance the enantioselectivity of the reaction through favoring the selective formation of the (E)-s-trans conformer in which steric interactions are minimized.

Scheme 10. The Michael reaction between aldehydes and nitroalkenes catalyzed by trans-hydroxyprolinamide 36.
On the other hand, tripeptide 38a has been demonstrated to be, up to date, the best performing catalyst in this transformation, being able to catalyze the transformation in a remarkably low 1 mol% catalyst loading (Scheme 11) [180]. The reaction can be carried out on a remarkably wide set of aldehyde donors and nitroalkene Michael acceptors, including the highly challenging nitroethylene [181] and also α,β- and β,β-disubstituted nitroalkenes [182,183]. In addition, the reaction proceeds under almost equimolar amounts of both Michael donor and acceptor, in deep contrast with most reported methodologies that typically required a large excess of the nitroalkene. Several key elements are required for the high performance of this catalyst. On one hand, the presence of the secondary pyrrolidine from the initial d-proline residue is crucial for both activity and enantioselectivity, and the configuration of the other two L-amino acid residues has also been recognized as the matched combination that provides the highest enantiocontrol [184]. On the other hand, the terminal carboxylic acid moiety of the final glutamic acid residue was also identified to be key for both activity and enantioselectivity [185]. Interestingly, reducing the size of this final carboxylate-containing side chain (from glutamic acid to aspartic acid) also led to a slight decrease in yield and enantioselectivity. A series of in-depth mechanistic studies [186,187,188,189,190,191,192] indicate that this carboxylic acid moiety is engaged in H-bonding interactions with the nitroalkene during the Michael addition between the intermediate enamine and the nitroalkene, but this acidic moiety is also crucial for promoting the fast protonation of the nitronate intermediate formed after the conjugate addition step. This favors catalyst turnover and leaves the C-C bond-forming step as the stereodefining event in the catalytic cycle [193]. Further studies in this area by other authors have shown that other structurally related tripeptides [194,195,196] or smaller dipeptides [197,198] also can catalyze this reaction with success.
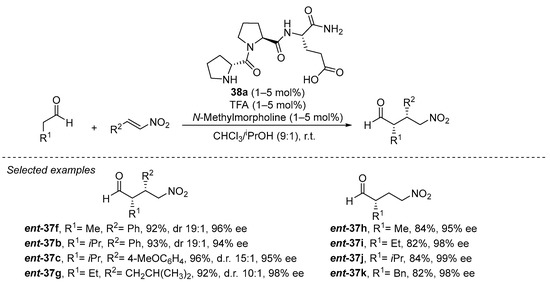
Scheme 11. The D-Pro-Pro-Glu-NH2 (38a)-catalyzed Michael reaction between aldehydes and nitroalkenes.
As an interesting variant, catalyst 38b, in which steric bulk at C4 of the active proline residue has been increased through the introduction of two methyl substituents, is an outstanding catalyst for the same Michael reaction, providing the opposite anti diastereoisomer with excellent yield and enantioselectivity (Scheme 12) [199]. The basis for this change in diastereoselectivity relies on the change in reactive conformation of the enamine intermediate, which in this case adopts a s-cis conformation in order to avoid steric clash between the alkene moiety and the two methyl substituents.
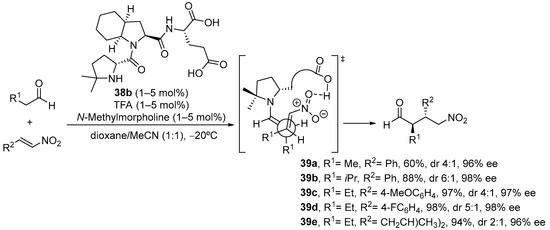
Scheme 12. The tripeptide 38b-catalyzed Michael reaction between aldehydes and nitroalkenes providing anti diastereoisomers.
The use of acyclic ketones as Michael donors typically makes use of primary amines as catalysts in order to favor the enamine intermediate with a lower degree of steric congestion compared to the situation when secondary amines are used. In this field, bifunctional primary amine/thioureas have gained a prominent position among the different systems reported to promote the Michael reaction between ketones and nitroalkenes. For example, 1,2-diphenylethylenediamine-based thiourea 40a has demonstrated its proficiency in this transformation with both acetone and other substituted acyclic ketones [200,201], in the latter case leading to the diastereoselective formation of the anti γ-nitro ketone diastereoisomer with high enantioselectivity (Scheme 13). This is explained in terms of the preferential participation of the Z enamine intermediate that avoids destabilizing steric interactions between the two alkyl substituents across the C=C bond. Moreover, in this case the reaction was also found to be completely regioselective, providing α-branched adduct 41e and without observing the formation of the potential regioisomer arising through the competitive formation of an unsubstituted enamine intermediate upon condensation of the substrate with the catalyst. Further progress in this field has evolved into a wide variety of structurally related primary amine/thiourea catalysts that also perform well in this transformation [144].
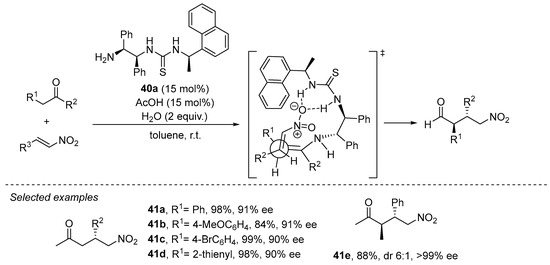
Scheme 13. The Michael reaction between acyclic ketones and nitroalkenes catalyzed by primary amine/thiourea 40a.
The same situation shows up when α,α-disubstituted aldehydes are to be used as Michael donors [202], in which the problems associated with the high degree of steric congestion around the nucleophilic carbon of the enamine are circumvented through the use of a primary amine catalyst instead of the archetypical pyrrolidine-based secondary amines. A particularly efficient approach comprises the use of bifunctional trans-cyclohexanediamine-derived thiourea 42a (Scheme 14) [203]. As can be seen in this scheme, this reaction performs excellently for a variety of situations, including challenging β-alkyl substituted nitroalkenes and also functionalized aldehydes, providing generally excellent yields and stereocontrol. It should also be pointed out that this catalyst is also particularly efficient in the Michael reaction between acyclic ketones and nitroalkenes [204].
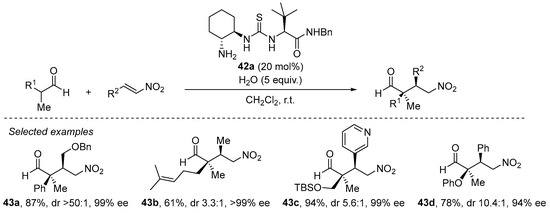
Scheme 14. The Michael reaction between α,α-disubstituted aldehydes and nitroalkenes catalyzed by primary amine/thiourea 42a.
This entry is adapted from the peer-reviewed paper 10.3390/catal13071091
This entry is offline, you can click here to edit this entry!
