Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Immune checkpoint inhibitor (ICI) therapy has revolutionized the treatment of many cancer types, including head and neck cancers (HNC). When checkpoint and partner proteins bind, these send an “off” signal to T cells, which prevents the immune system from destroying tumor cells. Immune checkpoint inhibitor (ICI) therapy has revolutionized the treatment of many cancer types, including head and neck cancers (HNC).
- immune checkpoint inhibitor
- T-cells
1. Introduction
The immune system is a dynamic and equipped mechanism, an intricate system of “recognition” and “on-off” switches. Unfortunately, cancers utilize this system to enable growth and escape. The role of the immune system in tumor regulation is particularly evident in the immunocompromised. Iatrogenic solid organ transplant, diabetes, autoimmunity requiring immunosuppressive therapy, HIV and hemoproliferative malignant disease or disorders and aging, are all associated with an increased risk of developing head and neck cancer (HNC) and worse outcomes [1,2,3,4,5,6,7,8,9,10,11]. Proliferating tumors utilize many forms of immunosuppression to tip the balance of immunoediting toward tumor progression [12]. Identifying therapies capable of shifting this balance back toward immunosurveillance should play an integral role in reducing morbidity- and mortality-associated HNC.
HNC, the sixth most common group of malignancies worldwide, results in 680,000 new cases annually, with squamous cell carcinoma (SCC) being the most common [13,14,15]. The incidence of HNC is increasing due to a range of factors including smoking, alcohol, human papillomavirus (HPV) infection and extended life expectancy [16].
Despite the vital role played by traditional therapies for HNSCC, namely surgery, radiotherapy and chemotherapy, prognosis remains poor and survival remains correlated to stage, with a 5-year survival rate of 50–60% and more than 60% presenting in the advanced stage [17,18]. More than 50% of HNSCCs have tumor recurrence and metastasis in less than 3 years [19]. Targeting the epidermal growth factor receptor (EGFR) was hailed a paradigm shift in personalizing HNSCC treatment, with the monoclonal antibody cetuximab demonstrating promise [20,21]; however, this has since demonstrated limited efficacy [22].
Compared with traditional therapies, new immunotherapy agents, namely antibodies targeting the PD-1/PD-L1 system, so-called immune checkpoint inhibitors (ICI) provide improved efficacy and comparatively lower toxicity for patients with advanced HNSCC [23,24,25,26]. KEYNOTE-048 (NCT02358031), a randomized open-label phase 3 study comparing the humanized monoclonal antibody pembrolizumab (Keytruda) targeting PD-1 alone or in conjunction with chemotherapy (platinum and 5-fluorouracil) against cetuximab with chemotherapy, demonstrated overall survival improvement in both treatment arms over standard-of-care therapy in recurrent or metastatic HNSCC [27]. Pembrolizumab was subsequently approved as a first-line therapeutic drug for patients with metastatic, unresectable and recurrent HNSCC. Unfortunately, the objective response rate (ORR) of pembrolizumab (or nivolumab/Optivo) in HNSCC is only 15%, with short-term durability [28,29]. In addition, immune-related adverse events (irAEs) secondary to immunotherapy treatment were identified in over 50% of patients, impacting clinical outcomes [30], with adverse-event-associated mortality evident in 0.3–1.3% of patients [31]. Common irAEs include gastrointestinal, dermatologic and endocrine toxicities, more specifically dermatitis, rash, nausea/vomiting, fever, headache, myalgia, hypothyroidism and fatigue [32]. Rarely, irAEs can be severe, resulting in carditis, nephritis, hepatitis, pneumonitis, gastrointestinal perforation and severe hematological dysfunction [33]. irAEs in ICI therapy have been associated with benefits, namely improvements in PFS, OS and ORR [34,35,36,37]. Consequently, balancing immunotherapy de-escalation or commencement of immunosuppressive therapy against a sub-optimal oncological outcome can be difficult.
Predictive biomarkers may be the key to identifying patients at risk of irAEs. To date, circulating blood counts and ratios, autoantibodies and autoantigens, microRNAs, gastrointestinal microbiome, T-cell diversification and expansion and cytokines are all being investigated; however, they remain to be validated for clinical use [38].
Biological, etiological, phenotypic and clinical heterogeneities characterize HNSCC and challenge the development of personalized medicine. However, poor survival, significant morbidity and compromised quality of life emphasize the requirement for innovative therapy. Immunoediting is the process through which the immune system can promote and constrain tumor development [39].
2. Immune Checkpoint Inhibitor Targets and Therapies
A successful objective ICI response revitalizes the immune system to recognize and target cancer cells. The roles of known key immune checkpoints CTLA-4, PD-1 and LAG-3 are summarized in Figure 1.
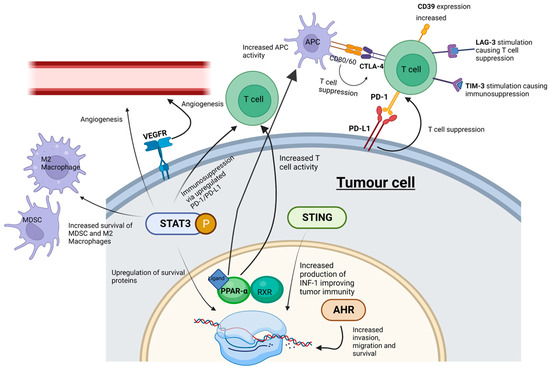
Figure 1. ICI and SMI actions within the tumor microenvironment. Whilst ICIs influence cell signaling at cell surface receptors, SMIs can interact with “upstream” intracellular signaling pathways-potentially playing a more effective role in abrogating tumor cell progression. MDSC, myeloid-derived suppressor cells; M2 macrophages, pro-tumorigenic macrophages; STING, stimulator of interferon genes; PPAR-α, peroxisome proliferator-activated receptor-α; AHR, aryl hydrocarbon receptor; STAT3, signal transducer and activator of transcription 3; P, phosphorylation of STAT3. Created with BioRender.com.
2.1. CTLA-4 and PD-1/PD-L1
CTLA-4 (cytotoxic T-lymphocyte associated protein 4, also known as cluster of differentiation 152, CD152) and programmed cell death protein 1 (PD-1) (and its ligands PD-L1 and PD-L2) are immune checkpoints targeted by humanized antibodies for the treatment of HNSCC. CTLA-4 is bound by ipilimumab (Yervoy), whereas PD-1 is targeted by pembrolizumab and nivolumab [32]. The antibodies atezolizumab (Tecentriq), durvalumab (Imfinzi) and avelumab (Bavencio) have also been approved as inhibitors of PD-L1 [33]. Both checkpoints regulate different stages of the immune response. CTLA-4 is considered the “leader” of the immune response and prevents the stimulation of autoreactive T-cells in the initial stage of naïve T-cell activation, whereas PD-1 is thought to regulate previously activated T-cells at the later stages of the immune response [32].
CTLA-4 is a homolog of CD28, but unlike CD28, CTLA-4 activation has an immunosuppressive effect opposite to the stimulatory effect of CD28 and the T-cell receptor (TCR) [40]. The binding of CD80/CD86 on antigen-presenting cells to CTLA-4 on T-cells in the tumor microenvironment suppresses the immune system, enabling tumor proliferation [41]. PD-1’s interaction with PD-L1 and PD-L2 has an immunosuppressive effect [41]. PD-L1 and PD-L2 are expressed by a range of tumors including HNSCC [42]. Critically, increased PD-1 levels serve as a biomarker for T cell exhaustion; this state of exhaustion is linked to T-cell dysfunction, which can facilitate tumor proliferation [43]. PD-L1′s interaction with PD-1 has an immunosuppressive effect, thus protecting cancer cells from lysis by activated T-cells [44].
Despite ICI therapy demonstrating survival advantage, comparatively few patients develop an effective response, the durability of which attenuates with acquired tumor resistance. Acquired resistance leads to tumor progression, and both arms of the immune system, innate and adaptive, can play a critical role in this change. Mechanisms of resistance to immunotherapy can be either intrinsic (tumor cell-mediated) or extrinsic (processes associated with T-cell activation) and shift the balance of immunomodulation towards tumor proliferation. Intrinsic resistance can include the downregulation of antigen-presenting machinery (APM) [45], the up-regulation of signaling pathways promoting T-cell exhaustion [46], the expression of multiple checkpoint inhibitors to mitigate T-cell activation [47], changes in tumor cell DNA repair, damage and genomic instability [48] and altered kinase signaling pathways [49]. Extrinsic resistance involves the complex interplay between tumor cells and the tumor microenvironment and its ability to regulate phenotypical characteristics of immune cells, especially TANs, TAMs, Tregs, MDSCs, T-cells, their associated regulatory cytokines and signaling pathways and a newly identified player, NETs [50,51,52,53,54,55,56].
Despite the clear improvements in overall survival due to immune checkpoint therapy, such treatments have limitations. For example, since CTLA-4 prevents the stimulation of autoreactive T-cells, inhibiting CTLA-4 can lead to grade 3 or 4 autoimmune-related adverse effects in 10–15% of patients [57]. Immune checkpoint immunotherapies are also associated with low response rates. For example, pembrolizumab has a response rate of only 15% in HNSCC [58].
To improve therapeutic failure and overcome immunotherapy resistance, significant energy is being invested in exploring biomarkers to predict clinical response and combinational therapies or changes in adjuvant delivery of immunotherapy to increase success rates. Biomarkers that have shown potential to determine improved clinical response in HNSCC include the tumor mutational burden, CCND1 amplification (CCND1 encodes cyclin D1, which regulates the retinoblastoma protein activity and cell-cycle progression), PD-1, IFN-γ, tumor-infiltrating lymphocytes (TILs) and cancer-associated fibroblasts (CAFs), CTLA-4, exosomes, CXCL, MTAP and SFR4/CPXM1/COL5A1 molecules [25,59,60,61,62,63,64,65,66,67,68,69].
Clinical trials exploring combinational immunotherapy in HNSCC are underway. The phase 3 randomized trial CheckMate 651 NCT02741570), which compared nivolumab and ipilimumab against EXTREME (platinum/5-fluorouracil/cetuximab) for R/M HNSCC, was unsuccessful in demonstrating OS improvement, although there was an association between elevated CPS and OS and durable response [70]. Other combination ICI therapy clinical trials have been largely unsuccessful (Table 1).
Table 1. Combination ICI Therapy Clinical Trials in HNSCC.
| Target | Combination | Phase | Trial | Intent | Outcome |
|---|---|---|---|---|---|
| PD-1, CTLA-4 | Nivolumab, Ipilimumab | 3 | NCT027441570 (CheckMate 651) [71] | Combination nivolumab + ipilimumab vs. EXTREME Regime (platinum/5-fluorouracil/cetuximab) for R/M HNSCC | Failed endpoint (OS). No difference between dual ICI blockade and EXTREME arm. Improvement in dual ICI arm if CPS > 20 (ns) |
| PD-L1, CTLA-4 | Durvalumab, Tremelimumab | 3 | NCT02551159 (KESTRAL) [72] | Combination durvalumab + tremelimumab vs. duravalumab monotherapy vs. SOC CT in R/M HNSCC | Results pending |
| PD-1, CTLA-4 | Nivolumab, Ipilimumab | 2 | NCT02823574 (CheckMate 714) [73] | Combination nivolumab + ipilimumab vs. nivolumab + ipilimumab placebo in R/M HNSCC | Failed ORR and OS endpoints. Subpopulation assessment ongoing. |
| PD-L1, CTLA-4 | Durvalumab, Tremelimumab | 3 | NCT02369874 (EAGLE) [74,75] | Combination durvalumab + tremelimumab vs. durvalumab monotherapy vs. SOC in R/M HNSCC | Failed to meet primary OS improvement endpoint |
Concurrent neoadjuvant and adjuvant delivery of ICIs has recently demonstrated benefits in surgically resectable advanced melanoma (Stage IIIB to IVC). In a recently completed Phase 2 randomized study (NCT03698019), neoadjuvant-adjuvant delivery of pembrolizumab was compared to an adjuvant alone in demonstrating an event-free survival of 72% in the neoadjuvant-adjuvant group compared to 49% in the adjuvant group after 2 years [76].
2.2. LAG-3
LAG-3 is expressed on activated human T-cells and natural killer cells and plays a similar role in T-cell regulation to CTLA-4 and PD-1 [77]. LAG-3 may represent an intrinsic resistance mechanism to PD-1 inhibitors due to its synergistic co-expression with PD-1 on exhausted T-cells [77]. To combat resistance, the FDA-approved drug opdualag® (combined LAG-3 and PD-1 inhibitor) became a first-line treatment for unresectable or metastatic melanoma in March 2022 [77]. Opdualag has shown success in clinical trials, more than doubling progression-free survival compared to melanoma patients treated with nivolumab alone [78].
2.3. Tim-3 and CD39
T cell immunoglobulin and mucin domain-containing protein 3 (Tim-3) is a co-inhibitory receptor expressed on IFN-γ-producing T-cells Tim-3. Studies by Liu et al. showed that Tim-3 is linked to immunosuppression in HNSCC and that targeting Tim-3 (with monoclonal antibodies) can enhance the anti-tumor immune response by reducing Tregs in HNSCC [79]. Similarly, the expression of the cell-surface ectonucleosidase CD39 in HNSCC positively correlates with tumor stage and predicts poor prognosis [80]. There are no approved inhibitors of Tim-3 or CD39, and opdualag has not yet been approved for HNSCC.
This entry is adapted from the peer-reviewed paper 10.3390/ijms241411695
This entry is offline, you can click here to edit this entry!
