Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Medical imaging techniques, including X-ray mammography, ultrasound, and magnetic resonance imaging, play a crucial role in the timely identification and monitoring of breast cancer. However, these conventional imaging modalities have their limitations, and there is a need for a more accurate and sensitive alternative. Microwave imaging has emerged as a promising technique for breast cancer detection due to its non-ionizing, non-invasive, and cost-effective nature.
- microwave imaging
- breast imaging
- microwave antenna
- antenna array
1. Breast Imaging Techniques
Conventional medical imaging modalities, such as X-ray mammography, ultrasound, MRI, CT, and positron emission tomography (PET), play a pivotal role in detecting breast cancer. X-ray mammography is commonly employed as the first line of defense due to its simple operation, high resolution, and high repeatability. However, it may not be a suitable option for all women, particularly those with dense breast tissue or who are pregnant [1]. Ultrasound imaging is a safe alternative and more convenient for high-risk patients. Still, it may not reveal breast lesions in adipose tissue and has a relatively low detection rate for malignant tumors [2]. MRI is a sensitive imaging technique that is particularly effective in detecting tumors in dense breast tissue but has limitations due to its cost, magnetic field exposure, and the noise generated during the procedure [3]. Breast CT examination provides a detailed view of breast tissue but is limited by its high radiation exposure and cost [4]. PET imaging is a powerful method for detecting breast cancer. Still, it has limitations due to its unsuitability for early stage tumor detection, the potential false positives in young patients, and the need for simultaneous use with CT imaging and its associated radiation exposure and expense [5].
MBI has been proposed as a complementary modality for early breast cancer diagnosis to overcome some limitations of conventional medical imaging modalities. Researchers aim to integrate this innovative imaging technique into routine clinical practice, providing patients with an accurate and reliable tool for detecting breast abnormalities at an early stage. Despite the significant progress in advancing MBI technologies, further research and development are necessary to establish connections between research labs, industrial partners, and patients who can benefit from these technologies.
2. Dielectric Properties of Breast Tissues
Microwave imaging (MWI) leverages electromagnetic (EM) waves ranging from 300 MHz to 30 GHz, penetrating the breast tissue. As these waves traverse the tissue, they interact with its dielectric properties, including permittivity (𝜀𝑟, the capacity to store electrical energy) and conductivity (σ, the ability to conduct electrical energy). Normal and malignant tumor tissues exhibit distinct dielectric properties, causing EM waves to scatter and reflect in each tissue type at varying degrees. This scattering and reflection serve as the foundation for the microwave imaging of breast tumors.
As shown in Figure 1, the dielectric properties of breast tissue change with working frequencies, and it is a nonlinear dependency [6]. The Debye and Cole–Cole models are commonly used to simulate biological tissues. The Debye model can be defined as follows [7]:

where 𝜀∞ denotes the permittivity, and its value strongly corresponds to the water content of the tissue, 𝜀𝑠 represents the static permittivity, and 𝜏 denotes the relaxation time.
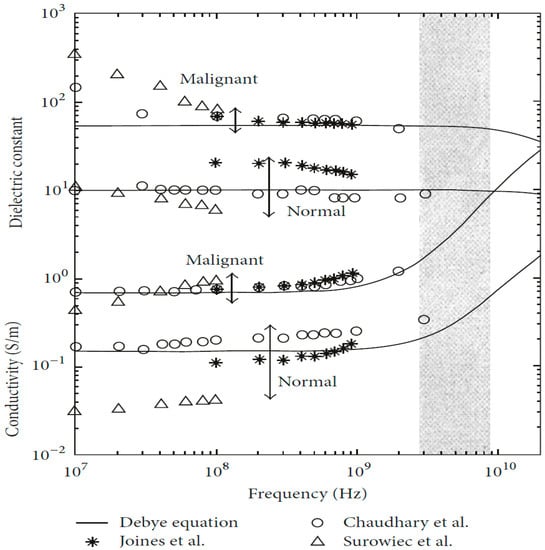
Figure 1. Dielectric property data for normal and malignant breast tissue [1]. Image from articles published under an open-access Creative Common CC BY license.
The Cole–Cole model can represent the complex dielectric constant of biological tissues [8].
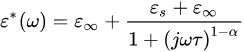
where 𝜀∗ denotes the complex dielectric, 𝜀𝑠 means the static frequency constant, 𝜀∞ represents infinite frequency constant, 𝜔 means the angular frequency, and 𝜏 denotes the time constant. The exponent parameter 𝛼 (0 < 𝛼 < 1) represents different spectral shapes. When 𝛼=0, the Cole–Cole model becomes the Debye model. When 𝛼>0, the relaxation time is increased.
The following empirical model can represent the relationship between the dielectric parameters and the moisture content model [9].
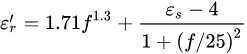
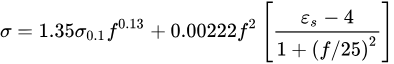
where 𝑓 is the frequency, 𝜎0.1=0.05, and 𝜀𝑠=8.5.
The dielectric properties of breast tissue exhibit a nonlinear dependence on working frequencies, which is commonly simulated using Debye or Cole–Cole models. These models consider the tissue’s water content and relaxation time. Experimental studies have shown that normal and malignant breast tissues have different electromagnetic responses due to differences in water and salt content [10][11]. Researchers have extensively studied the dielectric properties of biological tissues under the irradiation of electromagnetic waves with different frequencies, with findings indicating a significant difference in the dielectric properties of normal and tumor tissues [12][13]. Chaudhary et al. [14] discovered a substantial difference in the dielectric properties of normal and tumor tissues over the frequency range of 3–100 MHz. Joines et al. [15] replicated a similar study and confirmed Chaudhary’s findings. Gabriel et al. [10][11] reported their research findings on the characterization of biological tissues over the frequency range of 0.01 GHz to 20 GHz.
Furthermore, tissue at the infiltrating edge of the tumor has been found to have increased dielectric properties. Surowiec et al. [16] observed that the tissue at the infiltrating edge of the tumor had increased dielectric properties. Lazebnik et al. [12][13] studied the characterization of the dielectric properties of normal, malignant, and benign breast tissues over the frequency range of 0.5 GHz to 20 GHz. Halter et al. [17] conducted a similar measurement study on in vivo tissue, investigating the dielectric properties of breast tissues with and without tumor presence. Results showed negligible effects on the dielectric properties of tissues between excision and measurement.
Abas et al. [18] reported that the dielectric properties of tissues change significantly in the first few seconds after tissue excision. The authors investigated these effects based on ex vivo breast tissue measurement results. It is important to note that tissue dielectric properties can change significantly in the first few seconds after tissue excision due to temperature and water content changes.
Researchers have also developed breast phantoms based on measured dielectric properties of human breast tissue in the frequency range of 0.5–50 GHz, which have been tested using microwave imaging systems. Martellosio et al. [19] investigated the dielectric properties of breast tissues for the frequency range from 0.5 to 50 GHz. They employed Cole–Cole models for analyzing normal and tumorous tissues based on experimental measurements of 222 tissue samples from 53 patients aged 28 to 85. More recently, Meo et al. [20] developed three breast phantoms according to the dielectric properties of human breast ex vivo tissues in the frequency range of 0.5–50 GHz. The developed breast phantoms were tested using a microwave imaging system.
4. Microwave Breast Imaging Techniques
Microwave breast imaging (MBI) techniques can be classified into passive, hybrid, and active methods. Each method has its advantages and limitations. Passive methods rely solely on the radiation emitted by the body to create an image of the breast tissue. This approach does not require any external radiation sources. Passive methods are non-invasive but may have lower sensitivity. Hybrid microwave imaging combines microwave imaging with other imaging modalities such as ultrasound, MRI, or optical imaging. This combination of imaging techniques provides clinicians with more comprehensive and detailed information for accurate breast cancer diagnosis. Active methods utilize an external source of microwave radiation to probe the breast tissue. Active methods are more sensitive but need additional external radiation sources. Active MBI techniques such as microwave tomography (MT) and radar-based techniques have shown promise in accurately diagnosing breast cancer lesions. Radar-based methods use microwave radar to scan breast tissue in a non-invasive manner.
5. Microwave Breast Imaging Systems
A typical MBI system generally includes a microwave signal generator to generate microwave signals, microwave transmitters and microwave detectors, and a computer with an imaging program tool to analyze the measured microwave signals to reconstruct the target breast.
Microwave Antennas and Antenna Arrays for Breast Cancer Detection
Microwave antennas and antenna arrays are crucial in the MBI system. These antennas emit low-energy microwave signals that can penetrate breast tissue. As these signals interact with the tissue, they undergo scattering and are reflected to the antenna. These scattered signals contain valuable information about the internal structure of the breast tissue, which can be utilized for imaging. Multiple antennas are used in an antenna array configuration to enhance the spatial resolution and image quality of the MBI system. This approach has the potential to improve early detection rates and reduce patient discomfort that may be associated with traditional microwave imaging algorithms. However, the current performance of these antennas, in terms of their radiation capacity, gain, and bandwidth, requires improvement.
Monopole antennas offer a simple design among the different antennas used in the MBI system. They can be easily fabricated using printed circuit board (PCB) technology, making them cost-effective. Slot antennas, on the other hand, provide wideband performance and have a simple and low-cost design. UWB patch antennas are compact and low-profile, making them suitable for integration into wearable breast imaging systems. However, similar to other antenna types, their radiation capacity, gain, and bandwidth performance require improvement.
Regarding clinical trials and prototype testing, several groups have made significant progress in breast imaging. For example, the University of Bristol and Micrima Ltd. (Bristol, UK) conducted clinical tests on their MBI prototype called MARIA, involving 225 patients. They achieved a sensitivity rate of 76% [21]. Umbria Bioengineering Technologies (Rivotorto, Italy) conducted clinical trials on their prototype MammoWave, involving 58 patients, and achieved a sensitivity of 74% [22]. They also reported using support vector machine-based MBI for automatically identifying breast lesions, with an accuracy rate of 91%, a sensitivity rate of 84.4%, and a specificity rate of 97.2% [23]. Mitos Medical Technologies (Istanbul, Turkey) tested their MBI device called SAFE on 54 subjects, achieving a sensitivity rate of 63% [24]. Microwave Vision (MVG) Medical Imaging Department (Paris, France) clinically validated their MBI prototype called Wavelia on 24 patients, successfully distinguishing benign from malignant lesions with an accuracy rate of 88.5% [25].
Recently, researchers have started utilizing new technologies such as MTM [26], MTS [27], and AMC [28] in the development of microwave antennas. For instance, Hamza et al. [29] proposed an MTM microstrip patch antenna with AMC to enhance gain, achieving a gain of 10.61 dBi at 8.6 GHz. Mahmood et al. [30] designed UWB four-element multiple-input and -output (MIMO) wearable antennas to improve detection accuracy. With the advancement of the metasurface, MIMO, and deep learning technologies, new opportunities have emerged for researching and developing UWB MTS antennas to enhance detection accuracy and sensitivity further.
This entry is adapted from the peer-reviewed paper 10.3390/mi14071462
References
- Sollip, K.; Seungjun, L. Recent advances in microwave imaging for breast cancer detection. Int. J. Biomed. Imaging 2016, 2016, 5054912.
- Lee, J.M.; Arao, R.F.; Sprague, B.L.; Kerlikowske, K.; Lehman, C.D.; Smith, R.A.; Henderson, L.M.; Rauscher, G.H.; Miglioretti, D.L. Performance of Screening Ultrasonography as an Adjunct to Screening Mammography in Women Across the Spectrum of Breast Cancer Risk. JAMA Intern. Med. 2019, 179, 658–667.
- Leithner, D.; Moy, L.; Morris, E.A.; Marino, M.A.; Pinker, K. Abbreviated MRI of the breast: Does it provide value? J. Magn. Reson. Imaging 2019, 49, e85–e100.
- Yang, K.; Kwan, A.; Huang, S.; Boone, J. TH-C-332-03: Noise Power Properties of a Cone-Beam CT System for Breast Cancer Detection. Med. Phys. 2008, 35 Pt 26, 5317–5327.
- Suzuki, M.; Doi, H.; Hosoya, T.; Watanabe, Y. In vivo molecular imaging by positron emission tomography (PET) and its application to drug design and diagnosis. Biophysics 2004, 44, 265–270.
- Mustafa, S.; Abbosh, A.M.; Nguyen, P.T. Modeling human head tissues using fourth-order Debye model in convolution-based three-dimensional finite-difference time-domain. IEEE Trans. Antenna Propag. 2014, 62, 1354–1361.
- Lazebnik, M.; Okoniewski, M.; Booske, J.H.; Hagness, S.C. Highly accurate Debye models for normal and malignant breast tissue dielectric properties at microwave frequencies. IEEE Microw. Wirel. Compon. 2007, 17, 822–824.
- Kang, K.; Chu, X.; Dilmaghani, R.; Ghavami, M. Low-complexity Cole-Cole expression for modelling human biological tissues in (FD)2TD method. Electron. Lett. 2017, 43, 143–144.
- Zastrow, E.; Davis, S.K.; Lazebnik, M.; Kelcz, F.; Veen, B.D.V.; Hagness, S.C. Development of anatomically realistic numerical breast phantoms with accurate dielectric properties for modeling microwave interactions with the human breast. IEEE Trans. Bio-Med Eng. 2008, 55, 2792–2800.
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues. III. parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271–2293.
- Gabriel, G.C.; Gabriel, S. Compilation of the Dielectric Properties of Body Tissues at RF and Microwave Frequencies; Technical Report; Department of Physics, King’s College London: London, UK, 1996.
- Lazebnik, M.; McCartney, L.; Popovic, D.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Magliocco, A.; Booske, J.H.; Okoniewski, M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal breast tissue obtained from reduction surgeries. Phys. Med. Biol. 2007, 52, 2637–2656.
- Lazebnik, M.; Popovic, D.; McCartney, L.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Ogilvie, T.; Magliocco, A.; Breslin, T.M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys. Med. Biol. 2007, 52, 6093–6115.
- Chaudhary, S.S.; Mishra, R.K.; Swarup, A.; Thomas, J.M. Dielectric properties of normal & malignant human breast tissues at radio wave & microwave frequencies. Indian J. Biochem. Biophys. 1984, 21, 76–79.
- Joines, W.T.; Yang, Z.; Li, C.; Jirtle, R.L. The measured electrical properties of normal and malignant human tissues from 50 to 900 mhz. Med. Phys. 1994, 21, 547–550.
- Surowiec, A.J.; Stuchly, S.S. Dielectric properties of breast carcinoma and the surrounding tissues. IEEE Trans. Biomed. Eng. 1988, 35, 257–263.
- Halter, R.J.; Zhou, T.; Meaney, P.M.; Hartov, A.; Paulsen, K.D. The correlation of in vivo and ex vivo tissue dielectric properties to validate electromagnetic breast imaging: Initial clinical experience. Physiol. Meas. 2009, 30, S121–S136.
- Abas, S.; Camerin, H.; Sima, N.; Edward, S.; Tim, W. Study of the effects of changing physiological conditions on dielectric properties of breast tissues. ISRN Biomed. Imaging 2013, 2013, 894153.
- Martellosio, A.; Pasian, M.; Bozzi, M.; Perregrini, L.; Mazzanti, A.; Svelto, F.; Summers, P.E.; Renne, G.; Preda, L.; Bellomi, M. Dielectric properties characterization from 0.5 to 50 GHz of breast cancer tissues. IEEE Trans. Microw. Theory Tech. 2017, 65, 998–1011.
- Meo, S.D.; Matrone, G.; Pasian, M. Experimental validation on tissue-mimicking phantoms of millimeter-wave imaging for breast cancer detection. Appl. Sci. 2021, 11, 432.
- Shere, M.; Lyburn, I.; Sidebottom, R.; Massey, H.; Gillett, C.; Jones, L. MARIA® M5: A multicentre clinical study to evaluate the ability of the Micrima radio-wave radar breast imaging system (MARIA®) to detect lesions in the symptomatic breast. Eur. J. Radiol. 2019, 116, 61–67.
- Sani, L.; Vispa, A.; Loretoni, R.; Duranti, M.; Ghavami, N.; Sánchez-Bayuela, D.A.; Caschera, S.; Paoli, M.; Bigotti, A.; Badia, M.; et al. Breast lesion detection through MammoWave device: Empirical detection capability assessment of microwave images’ parameters. PLoS ONE 2021, 16, e0250005.
- Rana, S.P.; Dey, M.; Loretoni, R.; Duranti, M.; Sani, L.; Vispa, A.; Ghavami, M.; Dudley, S.; Tiberi, G. Radial Basis Function for Breast Lesion Detection from MammoWave Clinical Data. Diagnostics 2021, 11, 1930.
- Janjic, A.; Cayoren, M.; Akduman, I.; Yilmaz, T.; Onemli, E.; Bugdayci, O.; Aribal, M.E. SAFE: A Novel Microwave Imaging System Design for Breast Cancer Screening and Early Detection—Clinical Evaluation. Diagnostics 2021, 11, 533.
- Fasoula, A.; Duchesne, L.; Gil Cano, J.D.; Moloney, B.M.; Elwahab, S.M.A.; Kerin, M.J. Automated Breast Lesion Detection and Characterization with the Wavelia Microwave Breast Imaging System: Methodological Proof-of-Concept on First-in-Human Patient Data. Appl. Sci. 2021, 11, 9998.
- Shahmirzadi, N.V.; Tyagi, V.; Nguyen, J.; Kazemivala, R.; Nikolova, N.K.; Chen, C.H. Planar Array of UWB Active Slot Antennas for Microwave Imaging of the Breast. IEEE Trans. Antennas Propag. 2023, 71, 2946–2957.
- Alhawari, A.; Almawgani, A.; Hindi, A.T.; Alghamdi, H.; Saeidi, T. Metamaterial-based wearable flexible elliptical UWB antenna for WBAN and breast imaging applications. AIP Adv. 2021, 11, 015128.
- Zhang, Z.; Liu, T.; Cao, X.; Yang, H.; Jidi, L.; Gao, J. An integrated 2-bit metasurface array antenna with broadband low radar cross-section covering large incident angle space. IET Microw. Antennas Propag. 2022, 16, 367–377.
- Hamza, M.N.; Abdulkarim, Y.I.; Saeed, S.R.; Altıntaş, O.; Mahmud, R.H.; Appasani, B.; Ravariu, C. Low-Cost Antenna-Array-Based Metamaterials for Non-Invasive Early-Stage Breast Tumor Detection in the Human Body. Biosensors 2022, 12, 828.
- Mahmood, S.; Ishak, A.; Jalal, A.; Abbasi, Q. A bra monitoring system using a miniaturized wearable ultra-wideband MIMO antenna for breast cancer imaging. Electronics 2021, 10, 2563.
This entry is offline, you can click here to edit this entry!
