Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Bacterial Extracellular Vesicles (BEVs) possess the capability of intracellular interactions with other cells, and, hence, can be utilized as an efficient cargo for worldwide delivery of therapeutic substances such as monoclonal antibodies, proteins, plasmids, siRNA, and small molecules for the treatment of neurodegenerative diseases (NDs). BEVs additionally possess a remarkable capacity for delivering these therapeutics across the blood–brain barrier to treat Alzheimer’s disease (AD).
- bacterial extracellular vesicles
- therapeutics
- neurodegenerative disease
- Alzheimer’s disease
- nanocarriers
1. Offensive and Defensive Roles of Bacterial Extracellular Vesicles in Neurodegenerative Disease
BEVs generated from Pseudomonas aeruginosa were discovered in a recent study to induce inflammation and mortality of dopaminergic neurons in the substantia nigra [1][2][3]. Inflammation and mortality of dopaminergic neurons in the substantia nigra are two defining hallmarks of PD [1][4]. It was discovered that BEVs generated from Escherichia coli caused neuronal death and impaired memory in an AD mice model [3][4]. By inducing neuroinflammation and impairing neuronal function, BEVs may contribute to the pathogenesis of NDs, according to these findings [3][5].
In addition to their potential neurotoxicity [5], it has been demonstrated that BEVs serve a protective role in NDs [5]. Several studies have demonstrated, for instance, that BEVs can exert neuroprotective and immunomodulatory effects [5][6]. Specifically, it has been demonstrated that BEVs from commensal gut bacteria enhance cognitive function and reduce neuroinflammation in mouse models of NDs [6][7]. These results suggest that BEVs may also have therapeutic applications for the treatment of NDs [6][7]. The neurotoxicity of BEVs necessitates caution in their use as therapeutics, but their neuroprotective and immunomodulatory properties present opportunities for the development of novel treatments for NDs [6][8]. To thoroughly comprehend the mechanisms underlying the offensive and defensive roles of BEVs in NDs, additional research is required [5][8].
Recent research suggests that BEVs can also play a defensive function in NDs due to their neuroprotective and immunomodulatory properties [5][6]. Multiple studies have demonstrated, for instance, that BEVs derived from specific bacterial strains can improve neuronal survival and function in ND [7]. In the mouse model of AD, Haney et al. [9] found that BEVs from the probiotic Lactobacillus rhamnosus GG could reduce amyloid-beta (A) deposition and enhance cognitive function [10]. Few research studies have demonstrated that BEVs from Bifidobacterium infantis could reduce inflammation and oxidative stress in a mouse model of PD [11], resulting in enhanced motor function [6][7][11].
BEVs have been shown to possess immunomodulatory properties in the context of NDs, in addition to their neuroprotective effects [4][11]. By modulating the gut–brain axis [12], one study found that BEVs from Akkermansia muciniphila could reduce neuroinflammation and enhance cognitive function in a mouse model of PD [4][13]. Similarly, another study demonstrated that BEVs from Lactobacillus plantarum PS128 modulated microglial activity to enhance cognitive function and reduce neuroinflammation in an AD mouse model [11][14]. These studies suggest that BEVs may serve a dual role in NDs by possessing both offensive and defensive characteristics [4][14]. Some BEVs can induce neuroinflammation and impede neuronal function, whereas others can prevent diseases and modulate the immune system [6][10].
2. Critical Networks of Bacterial Extracellular Vesicles in the Microbiome–Gut–Brain Axis
The microbiome–gut–brain axis is a complex network of bidirectional communication between the gastrointestinal tract, the central nervous system (CNS), and the gut microbiota [7][15]. Recent evidence suggests that this axis regulates a variety of physiological and pathological processes, such as neuroinflammation and neurodegeneration [15][16]. The gut microbiota has a vast array of microorganisms inhabiting the human gastrointestinal tract, and it has been shown to influence brain function and behavior via multiple mechanisms [16]. These include the production of neurotransmitters and short-chain fatty acids, modulation of the immune system, and regulation of the hypothalamic–pituitary–adrenal axis [17][18]. Multiple NDs such as PD [17], AD [18][19], and MS have been linked to abnormalities in the gastrointestinal microbiota in their pathogenesis [19][20][21]. Additionally, it has been demonstrated that BEVs produced by intestinal microbiota can cross the blood–brain barrier (BBB) and directly affect the CNS function [11][20]. BEVs from the gut commensal Bacteroides fragilis have been shown to facilitate the differentiation and maturation of oligodendrocytes, which are essential to produce myelin in the CNS [22][23]. In a mouse model of AD, BEVs from Akkermansia muciniphila have been shown to protect against neuroinflammation and cognitive decline [12][13][23].
The above discussed results point out that the MGBA plays an important role in the pathogenesis of NDs, and that BEVs produced by intestinal microbiota may represent a novel drug delivery system for such conditions [24]. It has also been demonstrated that the gut microbiome can influence brain function and behaviors via multiple mechanisms, including the production of neurotransmitters, regulation of the immune system, and modulation of the gut–brain axis signaling pathways [25]. BEVs, which are produced by numerous bacteria in the microbiome of the gut, have been identified as potential mediators of this communication between the gut and the brain [22][25]. The effects of BEVs on the microbiome–gut–brain axis and their potential function in NDs have been studied and discussed in animal models [6]. In cell cultures and mouse models, BEVs from the gastrointestinal microbiome of PD patients were able to induce alpha–synuclein aggregation, which is a hallmark of PD pathology [4][11]. In a mouse model of AD, it was observed that BEVs from a specific gut bacterium, Akkermansia muciniphila, reduced neuroinflammation and enhance cognitive function [26].
Another study identified a group of BEVs produced by gut bacteria that could cross the BBB as a result, penetrating the brain, modulating the immune system [27], and potentially playing a significant role in NDs [5][16]. These studies indicate that BEVs can play a significant role in the communication between the gastrointestinal microbiome and the brain, and that their dysregulation may contribute to the development and progression of NDs [26][27]. The mechanisms underlying the effects of BEVs on the microbiome–gut–brain axis and their potential as therapeutic targets for NDs require additional study [16][26]. Among the NDs, a research study has shown that in AD brain, microglial activation contributes to amyloid-beta deposition and neuronal damage [28]. In addition, in PD few research studies have shown that in the brain T cells infiltrate the substantia nigra and promote neuroinflammation [29][30]. Moreover, in multiple sclerosis, few studies have shown that dysbiosis and gut-derived molecules contribute to neuroinflammation and disease progression [31][32][33].
3. Role of Bacterial Extracellular Vesicles in Neuroimmune System and Their Crosstalk
The neuroimmune system, which is made up of interactions between the neurological system and the immune system, is critical in NDs [34][35]. This neuroimmune system helps keep the homeostasis in balance. If this balance is distraught, it can lead to chronic inflammation, damage to neurons, and eventually NDs [28][29]. In terms of NDs, the neuroimmune system is made up of immune cells like microglia and astrocytes that become active when there is neuroinflammation [30]. When these cells become active, they release cytokines and chemokines that cause more inflammation and damage to neurons [30][31]. Peripheral immune cells, such as T cells and monocytes, can also promote neuroinflammation by crossing the BBB and entering the central nervous system [31][32].
Recent studies have shown how important the microbiome–gut–brain axis is in NDs and how it affects the neuroimmune system [36]. Dysbiosis, which is an imbalance in the gut microbiome, has been linked to the development of NDs [36][37]. This could be because small molecules from the gut, like lipopolysaccharides, affect the immune system [28][38]. The latest studies have looked at how BEVs and the neuroimmune system interact, which shows how BEVs might be able to change the immune response in NDs [5][27][38]. For example, BEVs made from the gut bacteria Bacteroides fragilis suppressed the immune response in a mouse model of multiple sclerosis [39]. It was found that the BEVs helped regulatory T cells grow; regulatory T cells are very important for calming down immune responses and preventing autoimmunity [40].
BEVs from the gut bacteria Akkermansia muciniphila were demonstrated to diminish neuroinflammation in a mouse model of PD [12][13]. It was found that the BEVs decreased the number of pro-inflammatory cytokines in the brain and increased the number of anti-inflammatory cytokines [4][38]. This suggests that BEVs have a neuroprotective effect. A study looked at how BEVs from the gut bacteria Bifidobacterium bifidum might affect the immune system in an AD animal model [10][33]. The researchers found that giving BEVs to the mice led to less inflammation in the brain and better brain function [33]. These studies show that BEVs may be able to change the immune response in NDs, which means they may be a good way to treat these diseases [5][36]. But more research is needed to fully understand the mechanisms behind these effects and to figure out the best ways to use BEVs as medicines.
4. Risk Factors of Bacterial Extracellular Vesicles in Autophagy–Lysosomal Pathway
The autophagy–lysosomal pathway (ALP) is a cellular process that gets rid of damaged organelles, misfolded proteins, and invading pathogens by breaking them down and recycling them [41][42]. Dysregulation of the ALP has been linked to ND. Studies of bacterial infections have shown that various types of EVs are released, including exosomes and microvesicles. The composition of the EV cargo can vary depending on the infection and cell type, and this can ultimately impact the host immune response and bacterial growth [43]. Figure 1 depicts the autophagy-related pathways that employs LC3 conjugation to membrane endocytic and phagocytic vesicles and their effect on EVs’ release.
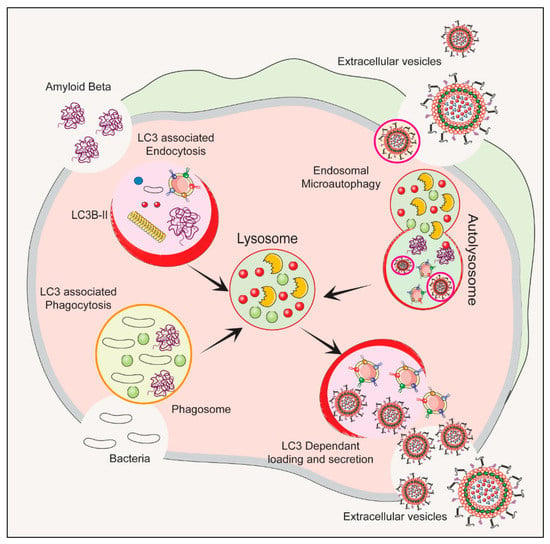
Figure 1. The process of initiation, nucleation and maturation of autophagy including the autophagosome formation and autophagy flux for the formation of autolysosome associated with LC3 Regulation of autophagy is dependent on the LC3 associated endocytosis, LC3 associated phagocytosis, Endosomal microautophagy and LC3 dependent loading and secretion of extracellular vesicles (EVs). The release of EVs and autophagy are two complementary mechanisms that cells use to eliminate amyloids and protein aggregates. EVs such as exosomes bud from late endosomes, which are themselves derived from multivesicular bodies (MVBs), which can either be released extracellularly or degraded in lysosomes. Autophagy is a cellular process in which cytosolic cargoes are sequestered into autophagosomes, which then fuse with lysosomes for degradation.
The ALP has three main types: macroautophagy, microautophagy, and chaperone-mediated autophagy, as shown in Figure 2. Autophagy or macroautophagy has been studied the most and it involves the formation of autophagosomes that engulf cytoplasmic parts and fuse with lysosomes to break them down [44][45].
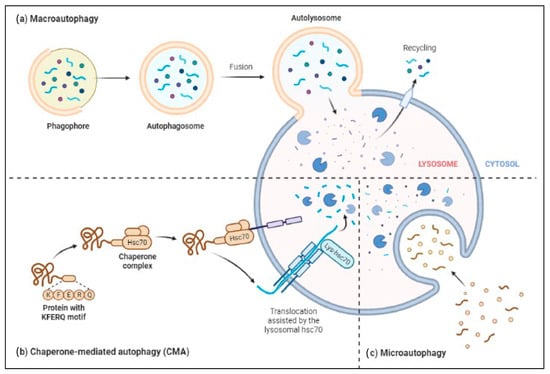
Figure 2. Three major types of autophagy–lysosomal pathways in NDs and other brain diseases are macroautophagy, chaperone-mediated autophagy, and microautophagy. Macroautophagy process degrades or eradicates the damaged cell organelles, unused protein, and toxic proteins by generating autophagosomes and fuse with lysosome. Chaperone-mediated autophagy degrades the unused proteins, and the misfolded proteins and intracellular toxic proteins are proteolytically degraded directly in lysosomes by translocating via the lumen of lysosomes. Microautophagy is a nonselective degradative process by accumulating the cytoplasmic contents directly to the lysosome by forming the endosomes.
ALP is very important in removing toxic proteins, like alpha-synuclein in PD, Aβ, and phospho tau in AD, from building up in the brain and trying to clear this toxic proteins when activated. Also, abnormal regulation of the ALP has been linked to the activation of inflammatory pathways and oxidative stress, both of which contribute to the development of NDs [32][46]. BEVs may be able to promote the ALP in different NDs, such as PD and AD [4][5][38]. Recent studies have shown that by controlling or promoting the ALP using BEVs, suggesting that BEVs could be a promising therapeutic target for treating NDs focusing on macroautophagy, chaperone-mediated autophagy, and microautophagy [33][47][48].
The ALP is very important for keeping cells in balance, and its malfunction has been linked to the development of several NDs. More research is needed to fully understand how the ALP works in NDs and to investigate the possibility that EVs, BEVs, or other external agents that could be used as a therapeutics to control the ALP are required for the present situation in the treatment of NDs [49][50][51]. As per the previous studies, EVs as brain delivery nanocarriers or other phytochemicals has been reported that may influence the autophagy–lysosomal pathway, in turn helping or keeping cells in balance by getting rid of damaged organelles and protein clusters [49][52][53]. Autophagy is a strictly regulated process that involves the creation of autophagosomes, which are double-membrane vesicles [42][54]. These vesicles take in the toxic cytoplasmic materials and send them to lysosomes by fusing them to form autolysosome to clear or break down the engulfed proteins or organelles [55][56]. There are many hydrolytic enzymes in lysosomes that can break down the contents of autophagosomes into nutrients that can be used to make energy and change the shape of cells [57][58].
Studies have shown that BEVs can mess up the autophagy–lysosomal pathway, which makes it harder for cells to get rid of waste and causes toxic aggregates to build up [58][59]. For example, a recent study showed that BEVs made from Porphyromonas gingivalis, a pathogenic oral bacterium linked to AD, could stop autophagy by stopping lysosomes from becoming acidic and stopping autophagosomes from breaking down [59][60][61]. BEVs made from Bacteroides fragilis, a common gut bacterium that can change the immune system, could stop autophagy in dendritic cells by stopping the fusion of autophagosomes and lysosomes [16][33][61]. On the other hand, some studies have shown that BEVs may be playing a protective role in the autophagy–lysosomal pathway by assisting to make new lysosomes and speeding up autophagic flux [33][57][59]. For example, a recent study showed that BEVs made from Lactobacillus acidophilus, a probiotic bacterium with anti-inflammatory properties, could improve autophagy flux by increasing lysosomal biogenesis and promoting lysosomal acidification [62][63]. It was also found that BEVs made from Akkermansia muciniphila linked to better metabolic health could speed up the removal of misfolded proteins in a mouse model of PD by activating the autophagy–lysosomal pathway [13][63][64]. In ND, the connection between BEVs and the autophagy–lysosomal pathway is complicated and needs to be investigated more [13][63][65]. The possibility that BEVs could interfere with or improve this important way for cells to get rid of waste could have big effects on how ND start and how they can be treated.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11072056
References
- Jones, L.B.; Kumar, S.; Bell, C.R.; Peoples, V.A.; Crenshaw, B.J.; Coats, M.T.; Scoffield, J.A.; Rowe, G.C.; Sims, B.; Matthews, Q.L. Effects of Pseudomonas aeruginosa on Microglial-Derived Extracellular Vesicle Biogenesis and Composition. Pathogens 2019, 8, 297.
- Lee, E.J.; Choi, Y.; Lee, H.J.; Hwang, D.W.; Lee, D.S. Human neural stem cell-derived extracellular vesicles protect against Parkinson’s disease pathologies. J. Nanobiotechnol. 2022, 20, 198.
- Aires, I.D.; Ribeiro-Rodrigues, T.; Boia, R.; Ferreira-Rodrigues, M.; Girao, H.; Ambrosio, A.F.; Santiago, A.R. Microglial Extracellular Vesicles as Vehicles for Neurodegeneration Spreading. Biomolecules 2021, 11, 770.
- Yuan, Q.; Li, X.D.; Zhang, S.M.; Wang, H.W.; Wang, Y.L. Extracellular vesicles in neurodegenerative diseases: Insights and new perspectives. Genes Dis. 2021, 8, 124–132.
- Xia, X.; Wang, Y.; Zheng, J.C. Extracellular vesicles, from the pathogenesis to the therapy of neurodegenerative diseases. Transl. Neurodegener. 2022, 11, 53.
- Yang, Z.; Gao, Z.; Yang, Z.; Zhang, Y.; Chen, H.; Yang, X.; Fang, X.; Zhu, Y.; Zhang, J.; Ouyang, F.; et al. Lactobacillus plantarum-derived extracellular vesicles protect against ischemic brain injury via the microRNA-101a-3p/c-Fos/TGF-beta axis. Pharmacol. Res. 2022, 182, 106332.
- Kang, C.S.; Ban, M.; Choi, E.J.; Moon, H.G.; Jeon, J.S.; Kim, D.K.; Park, S.K.; Jeon, S.G.; Roh, T.Y.; Myung, S.J.; et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS ONE 2013, 8, e76520.
- Choi, J.; Kim, Y.K.; Han, P.L. Extracellular Vesicles Derived from Lactobacillus plantarum Increase BDNF Expression in Cultured Hippocampal Neurons and Produce Antidepressant-like Effects in Mice. Exp. Neurobiol. 2019, 28, 158–171.
- Haney, M.S.; Bohlen, C.J.; Morgens, D.W.; Ousey, J.A.; Barkal, A.A.; Tsui, C.K.; Ego, B.K.; Levin, R.; Kamber, R.A.; Collins, H.; et al. Identification of phagocytosis regulators using magnetic genome-wide CRISPR screens. Nat. Genet. 2018, 50, 1716–1727.
- Konieczna, P.; Groeger, D.; Ziegler, M.; Frei, R.; Ferstl, R.; Shanahan, F.; Quigley, E.M.; Kiely, B.; Akdis, C.A.; O’Mahony, L. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: Potential role for myeloid and plasmacytoid dendritic cells. Gut 2012, 61, 354–366.
- Kim, W.; Lee, E.J.; Bae, I.H.; Myoung, K.; Kim, S.T.; Park, P.J.; Lee, K.H.; Pham, A.V.Q.; Ko, J.; Oh, S.H.; et al. Lactobacillus plantarum-derived extracellular vesicles induce anti-inflammatory M2 macrophage polarization in vitro. J. Extracell. Vesicles 2020, 9, 1793514.
- Chelakkot, C.; Choi, Y.; Kim, D.K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.S.; Jee, Y.K.; Gho, Y.S.; et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450.
- Ashrafian, F.; Shahriary, A.; Behrouzi, A.; Moradi, H.R.; Keshavarz Azizi Raftar, S.; Lari, A.; Hadifar, S.; Yaghoubfar, R.; Ahmadi Badi, S.; Khatami, S.; et al. Akkermansia muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice. Front. Microbiol. 2019, 10, 2155.
- Huang, H.J.; Chen, J.L.; Liao, J.F.; Chen, Y.H.; Chieu, M.W.; Ke, Y.Y.; Hsu, C.C.; Tsai, Y.C.; Hsieh-Li, H.M. Lactobacillus plantarum PS128 prevents cognitive dysfunction in Alzheimer’s disease mice by modulating propionic acid levels, glycogen synthase kinase 3 beta activity, and gliosis. BMC Complement. Med. Ther. 2021, 21, 259.
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209.
- Wang, H.; Long, T.; You, J.; Li, P.; Xu, Q. Bibliometric Visualization Analysis of Microbiome-Gut-Brain Axis from 2004 to 2020. Med. Sci. Monit. 2022, 28, e936037.
- Claudino Dos Santos, J.C.; Lima, M.P.P.; Brito, G.A.C.; Viana, G.S.B. Role of enteric glia and microbiota-gut-brain axis in parkinson disease pathogenesis. Ageing Res. Rev. 2023, 84, 101812.
- Kargbo, R.B. Microbiome-Gut-Brain Axis Modulation: New Approaches in Treatment of Neuropsychological and Gastrointestinal Functional Disorders. ACS Med. Chem. Lett. 2023, 14, 692–695.
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68.
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932.
- Jung, J.H.; Kim, G.; Byun, M.S.; Lee, J.H.; Yi, D.; Park, H.; Lee, D.Y.; Group, K.R. Gut microbiome alterations in preclinical Alzheimer’s disease. PLoS ONE 2022, 17, e0278276.
- Diaz-Garrido, N.; Badia, J.; Baldoma, L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J. Extracell. Vesicles 2021, 10, e12161.
- Chu, C.Q.; Yu, L.L.; Qi, G.Y.; Mi, Y.S.; Wu, W.Q.; Lee, Y.K.; Zhai, Q.X.; Tian, F.W.; Chen, W. Can dietary patterns prevent cognitive impairment and reduce Alzheimer’s disease risk: Exploring the underlying mechanisms of effects. Neurosci. Biobehav. Rev. 2022, 135, 104556.
- Sorboni, S.G.; Moghaddam, H.S.; Jafarzadeh-Esfehani, R.; Soleimanpour, S. A Comprehensive Review on the Role of the Gut Microbiome in Human Neurological Disorders. Clin. Microbiol. Rev. 2022, 35, e0033820.
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412.
- Ou, Z.; Deng, L.; Lu, Z.; Wu, F.; Liu, W.; Huang, D.; Peng, Y. Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer’s disease. Nutr. Diabetes 2020, 10, 12.
- Roig-Carles, D.; Willms, E.; Fontijn, R.D.; Martinez-Pacheco, S.; Mager, I.; de Vries, H.E.; Hirst, M.; Sharrack, B.; Male, D.K.; Hawkes, C.A.; et al. Endothelial-Derived Extracellular Vesicles Induce Cerebrovascular Dysfunction in Inflammation. Pharmaceutics 2021, 13, 1525.
- Rutsch, A.; Kantsjo, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179.
- Sterling, K.G.; Dodd, G.K.; Alhamdi, S.; Asimenios, P.G.; Dagda, R.K.; De Meirleir, K.L.; Hudig, D.; Lombardi, V.C. Mucosal Immunity and the Gut-Microbiota-Brain-Axis in Neuroimmune Disease. Int. J. Mol. Sci. 2022, 23, 13328.
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 2023, 46, 1–17.
- Patrycy, M.; Chodkowski, M.; Krzyzowska, M. Role of Microglia in Herpesvirus-Related Neuroinflammation and Neurodegeneration. Pathogens 2022, 11, 809.
- Zhang, F.; Jiang, L. Neuroinflammation in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2015, 11, 243–256.
- Zhu, G.; Guo, M.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Bifidobacterium breve intervention combined with environmental enrichment alleviates cognitive impairment by regulating the gut microbiota and microbial metabolites in Alzheimer’s disease mice. Front. Immunol. 2022, 13, 1013664.
- Cantoni, C.; Lin, Q.; Dorsett, Y.; Ghezzi, L.; Liu, Z.; Pan, Y.; Chen, K.; Han, Y.; Li, Z.; Xiao, H.; et al. Alterations of host-gut microbiome interactions in multiple sclerosis. EBioMedicine 2022, 76, 103798.
- Brochard, V.; Combadiere, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.M.; et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Investig. 2009, 119, 182–192.
- Haas-Neill, S.; Forsythe, P. A Budding Relationship: Bacterial Extracellular Vesicles in the Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2020, 21, 8899.
- Bicknell, B.; Liebert, A.; Borody, T.; Herkes, G.; McLachlan, C.; Kiat, H. Neurodegenerative and Neurodevelopmental Diseases and the Gut-Brain Axis: The Potential of Therapeutic Targeting of the Microbiome. Int. J. Mol. Sci. 2023, 24, 9577.
- Vanaja, S.K.; Russo, A.J.; Behl, B.; Banerjee, I.; Yankova, M.; Deshmukh, S.D.; Rathinam, V.A.K. Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell 2016, 165, 1106–1119.
- Ochoa-Reparaz, J.; Mielcarz, D.W.; Wang, Y.; Begum-Haque, S.; Dasgupta, S.; Kasper, D.L.; Kasper, L.H. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010, 3, 487–495.
- Eskandari, S.K.; Sulkaj, I.; Melo, M.B.; Li, N.; Allos, H.; Alhaddad, J.B.; Kollar, B.; Borges, T.J.; Eskandari, A.S.; Zinter, M.A.; et al. Regulatory T cells engineered with TCR signaling-responsive IL-2 nanogels suppress alloimmunity in sites of antigen encounter. Sci. Transl. Med. 2020, 12, eaaw4744.
- Zhu, Z.; Yang, C.; Iyaswamy, A.; Krishnamoorthi, S.; Sreenivasmurthy, S.G.; Liu, J.; Wang, Z.; Tong, B.C.; Song, J.; Lu, J.; et al. Balancing mTOR Signaling and Autophagy in the Treatment of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 728.
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 24, 24–41.
- Spencer, N.; Yeruva, L. Role of bacterial infections in extracellular vesicles release and impact on immune response. Biomed. J. 2021, 44, 157–164.
- Menzies, F.M.; Fleming, A.; Rubinsztein, D.C. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 2015, 16, 345–357.
- Yang, C.; Su, C.; Iyaswamy, A.; Krishnamoorthi, S.K.; Zhu, Z.; Yang, S.; Tong, B.C.; Liu, J.; Sreenivasmurthy, S.G.; Guan, X.; et al. Celastrol enhances transcription factor EB (TFEB)-mediated autophagy and mitigates Tau pathology: Implications for Alzheimer’s disease therapy. Acta Pharm. Sin. B 2022, 12, 1707–1722.
- Sarkar, S.; Malovic, E.; Harischandra, D.S.; Ngwa, H.A.; Ghosh, A.; Hogan, C.; Rokad, D.; Zenitsky, G.; Jin, H.; Anantharam, V.; et al. Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes. Neurotoxicology 2018, 64, 204–218.
- Xu, R.; Zhang, Y.; Chen, S.; Zeng, Y.; Fu, X.; Chen, T.; Luo, S.; Zhang, X. The role of the probiotic Akkermansia muciniphila in brain functions: Insights underpinning therapeutic potential. Crit. Rev. Microbiol. 2023, 49, 151–176.
- Gaurav, I.; Thakur, A.; Kumar, G.; Long, Q.; Zhang, K.; Sidu, R.K.; Thakur, S.; Sarkar, R.K.; Kumar, A.; Iyaswamy, A.; et al. Delivery of Apoplastic Extracellular Vesicles Encapsulating Green-Synthesized Silver Nanoparticles to Treat Citrus Canker. Nanomaterials 2023, 13, 1306.
- Thakur, A.; Ke, X.; Chen, Y.W.; Motallebnejad, P.; Zhang, K.; Lian, Q.; Chen, H.J. The mini player with diverse functions: Extracellular vesicles in cell biology, disease, and therapeutics. Protein Cell 2022, 13, 631–654.
- Panaro, M.A.; Benameur, T.; Porro, C. Extracellular Vesicles miRNA Cargo for Microglia Polarization in Traumatic Brain Injury. Biomolecules 2020, 10, 901.
- Go, V.; Bowley, B.G.E.; Pessina, M.A.; Zhang, Z.G.; Chopp, M.; Finklestein, S.P.; Rosene, D.L.; Medalla, M.; Buller, B.; Moore, T.L. Extracellular vesicles from mesenchymal stem cells reduce microglial-mediated neuroinflammation after cortical injury in aged Rhesus monkeys. GeroScience 2020, 42, 505–514.
- Sil, S.; Singh, S.; Chemparathy, D.T.; Chivero, E.T.; Gordon, L.; Buch, S. Astrocytes & Astrocyte derived Extracellular Vesicles in Morphine Induced Amyloidopathy: Implications for Cognitive Deficits in Opiate Abusers. Aging Dis. 2021, 12, 1389–1408.
- Jones, E.; Stentz, R.; Telatin, A.; Savva, G.M.; Booth, C.; Baker, D.; Rudder, S.; Knight, S.C.; Noble, A.; Carding, S.R. The Origin of Plasma-Derived Bacterial Extracellular Vesicles in Healthy Individuals and Patients with Inflammatory Bowel Disease: A Pilot Study. Genes 2021, 12, 1636.
- Wong, E.; Cuervo, A.M. Autophagy gone awry in neurodegenerative diseases. Nat. Neurosci. 2010, 13, 805–811.
- Iyaswamy, A.; Wang, X.; Krishnamoorthi, S.; Kaliamoorthy, V.; Sreenivasmurthy, S.G.; Kumar Durairajan, S.S.; Song, J.X.; Tong, B.C.; Zhu, Z.; Su, C.F.; et al. Theranostic F-SLOH mitigates Alzheimer’s disease pathology involving TFEB and ameliorates cognitive functions in Alzheimer’s disease models. Redox Biol. 2022, 51, 102280.
- Iyaswamy, A.; Vasudevan, K.; Jayaraman, S.; Jaganathan, R.; Thakur, A.; Chang, R.C.; Yang, C. Editorial: Advances in Alzheimer’s disease diagnostics, brain delivery systems, and therapeutics. Front. Mol. Biosci. 2023, 10, 1162879.
- Wu, S.; Shen, Y.; Zhang, S.; Xiao, Y.; Shi, S. Salmonella Interacts With Autophagy to Offense or Defense. Front. Microbiol. 2020, 11, 721.
- Keller, M.D.; Torres, V.J.; Cadwell, K. Autophagy and microbial pathogenesis. Cell Death Differ. 2020, 27, 872–886.
- Villageliu, D.N.; Samuelson, D.R. The Role of Bacterial Membrane Vesicles in Human Health and Disease. Front. Microbiol. 2022, 13, 828704.
- Chen, M.F.; Lu, M.S.; Hsieh, C.C.; Chen, W.C. Porphyromonas gingivalis promotes tumor progression in esophageal squamous cell carcinoma. Cell. Oncol. 2021, 44, 373–384.
- Di Gioia, S.; Daniello, V.; Conese, M. Extracellular Vesicles’ Role in the Pathophysiology and as Biomarkers in Cystic Fibrosis and COPD. Int. J. Mol. Sci. 2022, 24, 228.
- Li, M.; Lee, K.; Hsu, M.; Nau, G.; Mylonakis, E.; Ramratnam, B. Lactobacillus-derived extracellular vesicles enhance host immune responses against vancomycin-resistant enterococci. BMC Microbiol. 2017, 17, 66.
- Choi, J.H.; Moon, C.M.; Shin, T.S.; Kim, E.K.; McDowell, A.; Jo, M.K.; Joo, Y.H.; Kim, S.E.; Jung, H.K.; Shim, K.N.; et al. Lactobacillus paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp. Mol. Med. 2020, 52, 423–437.
- Kim, D.K.; Lee, J.; Kim, S.R.; Choi, D.S.; Yoon, Y.J.; Kim, J.H.; Go, G.; Nhung, D.; Hong, K.; Jang, S.C.; et al. EVpedia: A community web portal for extracellular vesicles research. Bioinformatics 2015, 31, 933–939.
- Cadwell, K. Crosstalk between autophagy and inflammatory signalling pathways: Balancing defence and homeostasis. Nat. Rev. Immunol. 2016, 16, 661–675.
This entry is offline, you can click here to edit this entry!
