Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Chlorogenic and isochlorogenic acids are naturally occurring antioxidant dietary polyphenolic compounds found in high concentrations in plants, fruits, vegetables, coffee, and coffee by-products. Coffee is particularly cultivated and produced in tropical and subtropical regions along the equator (the so-called “coffee belt”), where ideal growth is possible due to the constantly warm temperatures and humid climate without extreme weather fluctuations.
- novel foods
- coffee by-products
- chlorogenic acid
- CQA
1. Introduction
Coffee is one of the best-selling and most consumed beverages worldwide [1]. In Germany, the coffee consumption per person is four cups a day, which corresponds to 6.7 kg of coffee beans per year [2]. There are several thousand varieties of the Coffea genus, with Coffea arabica and Coffea canephora being the most important species. Coffee is particularly cultivated and produced in tropical and subtropical regions along the equator (the so-called “coffee belt”), where ideal growth is possible due to the constantly warm temperatures and humid climate without extreme weather fluctuations. The most important coffee producers include Brazil, Vietnam, Colombia, Indonesia, Ethiopia, and India [2][3][4]. In addition to the main ingredient, caffeine (1a), the purine alkaloids theophylline (1b) and theobromine (1c), the diterpenes kahweol (2), cafestol (3a), and 16-O-methylcafestol (3b), as well as the flavonoid epigallocatechin gallate (4) and the polyphenolic chlorogenic acids (5) are present in coffee; these are shown in Figure 1 [4][5][6].
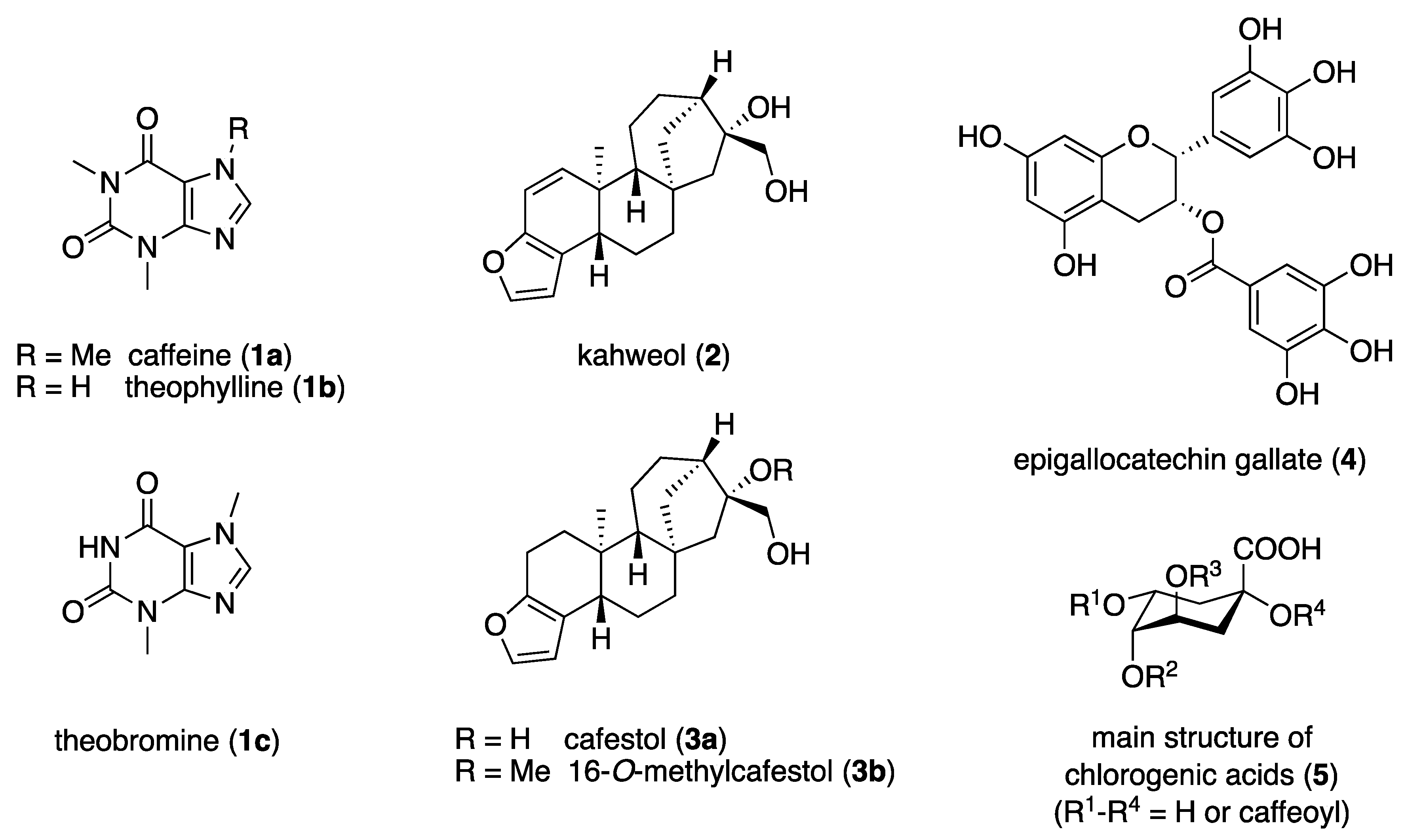
Figure 1. Major constituents of Coffea spp.
Due to the global climate crisis, the weather has changed to more extreme temperatures and less rainfall all over the planet. Hotter temperatures and extended dry periods in summer and extreme unusual frosts in winter have caused enormous coffee harvest losses over the last few years, which have also increased the price of coffee worldwide. Attempts are now being made to use all components of the coffee plant (the so-called “coffee by-products”) in addition to the coffee bean itself to increase sustainable coffee production and to reduce its carbon footprint.
Furthermore, the process of coffee production generates much waste (in the form of by-products) and wastewater. Especially with the many washing steps during production, large amounts of contaminated water with high carbon loading accumulate, which in turn represents a high environmental burden [7][8]. One of the most promising options to reduce the environmental impact of coffee production and make the process more sustainable is to utilize the resulting by-products by using the biologically active substances; for example, as functional food, novel food ingredients, or food supplements [8][9].
Before coffee by-products can be marketed in the food sector within the European Union (EU), it is of great importance that they acquire approval as novel foods. Novel foods are foods and/or food ingredients that are relatively new to the European market and therefore have no history of use as safe foods for human consumption [10]. The European legal framework for novel foods intends to protect human health and consumer interests. Regulation (EU) No. 2015/2283 defines “novel food” as any food that was not used for human consumption to a significant extent within the Union before 15 May 1997 [11][12]. Both the classification as traditional food from non-EU countries and the full application for authorization as a novel food require data of sufficient quantity and quality, including a description of the novel food, its manufacture, chemical analysis methods, and analytical and toxicological data to demonstrate that there is no safety risk to human health. The European Commission is responsible for the authorization of novel foods, which is often supported by the European Food Safety Authority (EFSA) since the EFSA carries out toxicological risk assessments to ensure (food) safety. Common coffee by-products, defined as any product derived from coffee production other than roasted coffee, are coffee flowers (blossoms), leaves, coffee cherry materials (husks, cascara, dried or fresh coffee cherries, and coffee pulp or mucilage), silver skin, parchment, green unroasted beans, and spent coffee grounds (Figure 2) [10][13][14][15].

Figure 2. Some coffee by-products (coffee flowers/blossoms, coffee leaves, ripe and unripe (green) coffee cherries).
2. Chlorogenic Acids
2.1. Structures, Properties, and Natural Occurrence
Originally, the naturally occurring esters of trans-configurated caffeic acid (6) and (–)-quinic acid (7) were known by the trivial name chlorogenic acid (CQA, singular). In 1846, Payen used the term “chlorogen acid” for the first time and isolated the crystalline potassium caffeine chlorogenate complex from green coffee (Coffea arabica) [16][17]. The name chlorogenic acid comes from the Greek χλωρός (khloros, light green) and γένος (ghenos, producing) and refers to the intense green color that results when chlorogenic acids are oxidized [2]. The origin of the name may also be due to a green color that appears when chlorogenic acid is treated with an aqueous solution of ammonia in the presence of atmospheric oxygen [18]. The prefix “chloro” is misleading in that the substances have no chlorine atoms in their chemical structure. The first time that pure chlorogenic acid was obtained in crystalline form with a melting point of 206–207 °C was in 1907, with evidence being provided by the formation of caffeic acid and quinic acid after alkaline hydrolysis [19]. Twenty-five years later, Freudenberg confirmed that chlorogenic acid is a caffeic acid–quinic acid conjugate [20].
Today, several structurally similar compounds belong to the class of chlorogenic acids (CQAs, plural), which also includes esters of other hydroxycinnamic acids such as ferulic acid (8) and p-coumaric acid (9) [21][22][23][24][25]. Chlorogenic acids are secondary metabolites and belong to the biologically active dietary polyphenols with a phenylpropanoid moiety. They are found in numerous plants, fruits, and vegetables [26] and play an important role as an intermediate in the biosynthesis of lignin [27][28]. Figure 3 shows an overview of the chemical structures of the main chlorogenic acids (10–13).
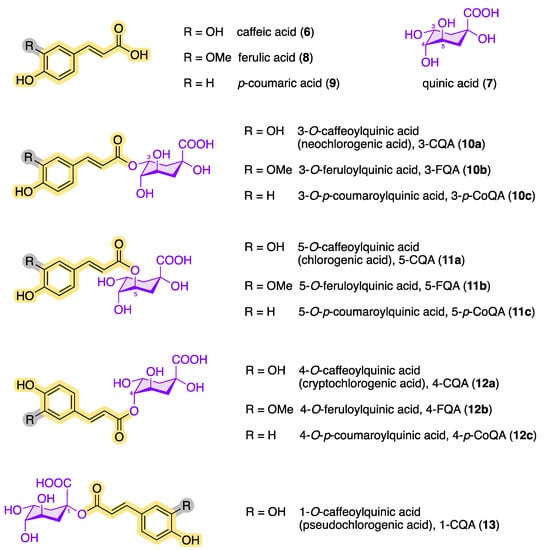
Figure 3. Chemical structures of quinic acid, hydroxycinnamic acids, and the most common naturally occurring CQAs (following the IUPAC numbering system).
It was not until 1932 that Fischer and Dangschat suggested that the substance isolated by Freudenberg must be 3-caffeoylquinic acid (3-CQA, 10a) [29]. According to today’s IUPAC nomenclature, the 3-CQA postulated by Fischer and Dangschat is 5-caffeoylquinic acid (5-CQA, 11a). Various publications on further structural analogues followed in the subsequent years.
To date, over 80 different chlorogenic acids have been identified exclusively in green coffee [30], and more than 400 CQAs are currently known [31]. The structural diversity of chlorogenic acids results from the fact that the hydroxycarboxylic acid has four hydroxy groups that are arranged differently in space. According to the IUPAC nomenclature, the two hydroxy groups at C3 and C4 of (–)-quinic acid (7) are arranged equatorially, while the OH group at position 5 is axial (see Figure 4) [32][33]. Each of these OH functions of (–)-quinic acid can form corresponding esters with the hydroxycinnamic acid derivatives 6, 8, and 9. In addition to the chlorogenic acids shown in Figure 3, other hydroxycinnamic acid–quinic acid conjugates are also known, e.g., those made from sinapinic acid or 3,4,5-trimethoxycinnamic acid and (–)-quinic acid (7) or multiple mixed esters such as caffeoyl-feruloylquinic acids (CFQAs) [7][15][34].
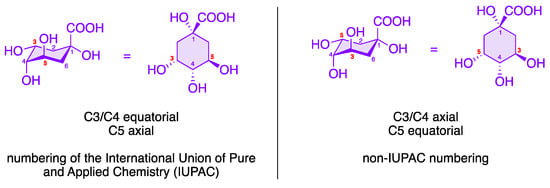
Figure 4. Numbering of the (–)-quinic acid fragment in CQAs.
Among the configurational/conformational/regio-isomers 3-CQA (10a), 4-CQA (12a), 5-CQA (11a) and 1-CQA (13), 5-CQA (11a) is the most prevalent chlorogenic acid in coffee [15][35]. The epimer 1-CQA (13) occurs in nature very rarely and in very small amounts. Parejo et al. were able to identify 1-CQA in fennel (Foeniculum vulgare) using LC-DAD-ESI-MS/MS as the chromatographic method [36].
In addition, the chlorogenic acids can be converted into one another by transesterification (Figure 5). The solvent used in the extraction process also has an impact on the transesterification reaction. For example, the chlorogenic acid 1-caffeoylquinic acid (13), which tends to be underrepresented in plants, can be formed via acyl migration being accelerated by an increased amount of water in the organic extractant or in the plant material [37][38][39]. Transesterification reactions are also possible with FGAs and p-CoGAs in order to obtain the other structural isomers.
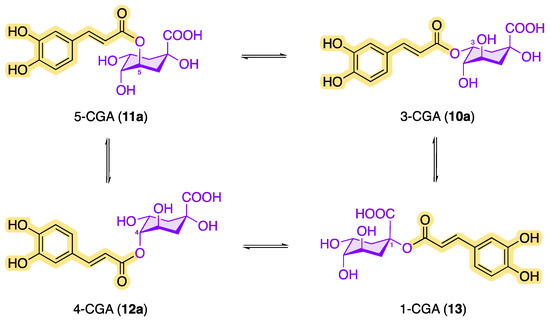
Figure 5. Conversion of CQAs by transesterification.
In addition to caffeine, chlorogenic acids occur as an ingredient in green and roasted coffee [40]. However, the content of chlorogenic acids in green coffee is greater than in roasted coffee [41][42][43][44]. During the roasting process, CQAs can be converted by dehydration into chlorogenic acid lactones such as 3-O-caffeoylquinic-1,5-γ-lactone (3-CGL, 14) and 5-O-caffeoylshikimic acid (dactylifric acid, 15), which results in a decreased amount of classic CQAs [29][45][46]. Figure 6 shows examples of possible roasted products based on monocaffeoylquinic acids.
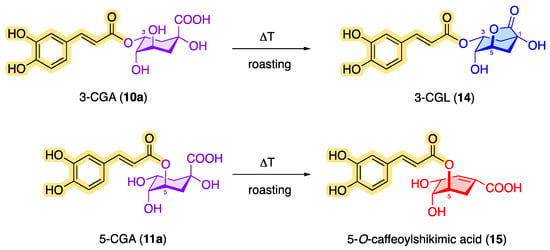
Figure 6. CQA products in roasted coffee: formation of 3-O-caffeoylquinic-1,5-γ-lactone (14) and 5-O-caffeoylshikimic acid (15).
The monocaffeoylquinic acids can be found in numerous plants, such as valerian (Valeriana officinalis) [47], sunflower (Helianthus annuus) [48], bamboo (Phyllo-stachys edulis) [49], heather (Calluna vugaris from Ericaceae) [50], lemon balm (Melissa officinalis) [51], nettle (Urtica dioica) [52], and Japanese honeysuckle (Lonicera japonica) [53], but also in the pulp of blueberries (Vaccinium corymbosum) [54], apples (Malus domestica) [55], grapes (Vitis vinifera) [56], eggplant (Solanum melongena) [57], peaches (Prunus persica) [58] and dried plums (Prunus domestica) [59], and in the roots of chicory (Cichorium intybus) [60]. Chlorogenic acid (11a), cryptochlorogenic acid (12a), and neochlorogenic acid (10a) can also be detected in the leaves of the African mallow (Hibiscus sabdariffa from Malvaceae) [61] and in walnuts (Juglans regia) [62].
Structures having more than one caffeic acid residue are called dicaffeoylquinic acids or isochlorogenic acids and can be found, e.g., in coffee and in plants of the family Asteraceae [63]. Prominent isochlorogenic acids are shown in Figure 7. The most abundant isochlorogenic acid in extracts from Indian pennywort (Centella asiatica from Apiaceae) and other plant sources is 3,5-DCQA (16a) [64][65]. In 1954, the dicaffeoylquinic acid 1,3-DCQA (16b) was isolated and characterized as the first 1-acyl quinic acid from the artichoke (Cyanara cardunculus) [21][66]. Also called cynarine, 1,3-DCQA (16b) has antioxidant and anticholinergic effects. Other isochlorogenic acids have also been found in the leaves of sweet potato (Ipomoea batatas) and white and green tea (Camellia sinensis) [67].
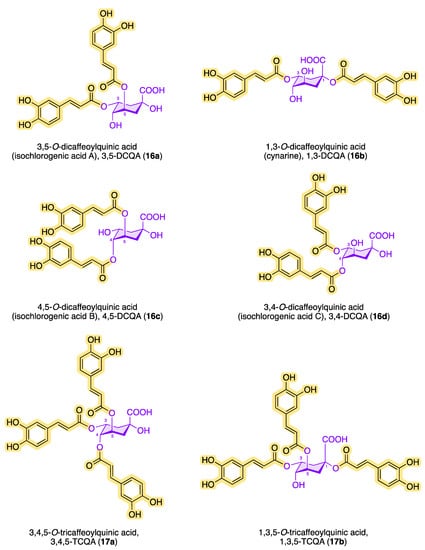
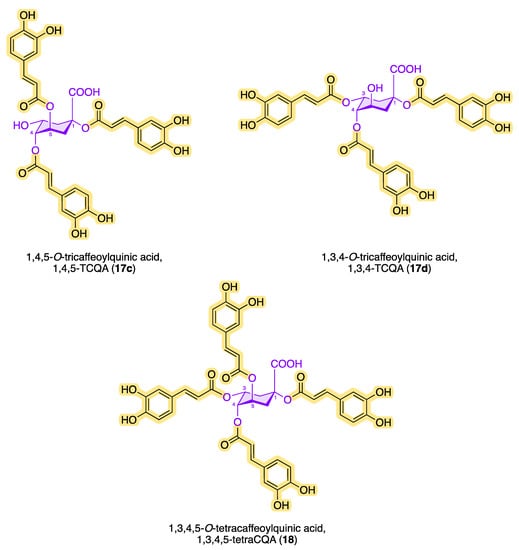
Figure 7. Structures of di-, tri- and tetra-caffeoylquinic acids.
Chlorogenic and isochlorogenic acids have demonstrated various effects in several studies. CQAs have antioxidant [68], antibacterial [69], antiviral [70], antidiabetic [71], neuroprotective [72][73], anti-inflammatory [74], and cytostatic effects [75][76]. CQAs have been used therapeutically in some clinical treatments as well, e.g., in the treatment of cardiovascular diseases [77] and arterial hypertension (high blood pressure) [78]. The broad scope of bioactivities and pharmacological applications have attracted much attention from research scientists. Novel synthetic chlorogenic-acid amide analogues have shown both higher chemical stability compared with classic chlorogenic acids and biological activity against the hepatitis C virus [79]. Several other chlorogenic acid derivatives have also been made synthetically accessible and have other promising properties; for example, they have an antifungal effect and effectively inhibit the HIV integrase/protease [80].
In addition to chlorogenic acid and isochlorogenic acid, there are other quinic acid conjugates with a higher number of caffeoyl residues. Figure 7 shows the chemical structures of known tri- and tetracaffeoylquinic acids. The ubiquitous occurrence of tricaffeoylquinic acids (TCQAs) in the plant kingdom has been known for a long time. Bates et al. characterized and identified 3,4,5-TCQA (17a) for the first time in 1983 from methanolic extracts of black-banded rabbitbrush (Chrysothamnus paniculatus of the Compositae family) [81].
In 1993, Agata et al. detected 1,3,5-TCQA (17b) in the fruits of the common cocklebur (Xanthium strumarium) [82]. A year earlier, Merfort reported the discovery of 1,4,5-TCQA (17c) from the flowers of Arnica montana and Arnica chamissonis by extraction with ethyl acetate [83]. In 2018, Liu et al. demonstrated the occurrence of 1,3,4-TCQA (17d) in Duhaldea nervosa chromatographically and spectroscopically [84]. Since then, the number of tricaffeoylquinic acids in different plants with a variety of biological activities has increased significantly. Kim et al. reported the interesting observation that sunlight significantly increases the amount of CQAs when isolating and quantifying 1,3,4-TCQA and 1,3,5-TCQA from Ligulara fischeri [85]. The antihyperglycemic effect of 3,4,5-TCQA was confirmed in a study with a water-soluble fraction of Brazilian propolis [86]. In 1994, Scholz et al. discovered 1,3,4,5-O-tetracaffeoylquinic acid (18) from the aerial parts of Pluchea symphytfolia [87], which showed significant inhibition on the growth of bacterial strains such as Escherichia coli, Bacillus subtilis, and Micrococcus luteus and had a weak anthelmintic effect in in vitro studies [88]. In addition to the TetraCQA (18) isolated by Scholz et al., cyclobutane structural analogues have also been found, which formally result from a pericyclic reaction via the [2+2]-cycloaddition between two neighboring caffeoyl residues [60]. It is particularly impressive that 1,3,4,5-O-tetracaffeoylquinic acid (18) inhibits Trypanosoma brucei RTPase Cet1 (TbCet1) extraordinarily effectively but is, on the other hand, necessary for the proliferation of procyclic cells [89]. Using a colorimetric high-throughput screening (HTS) assay, the inhibitory activity (IC50) of TetraCQA was found to be in the submicromolar range at 13 nM and is the most effective inhibitor compared with around 20 other TbCet1 inhibitors. Isochlorogenic acid A (16a) is also effective at inhibiting TbCet1, with an IC50 of 70 nM. The number of naturally occurring derivatives of the CQA family could be determined as follows: DCQA > TCQA > TetraCQA [7].
2.2. Biosynthetic Pathways and Totally Synthetic Approaches
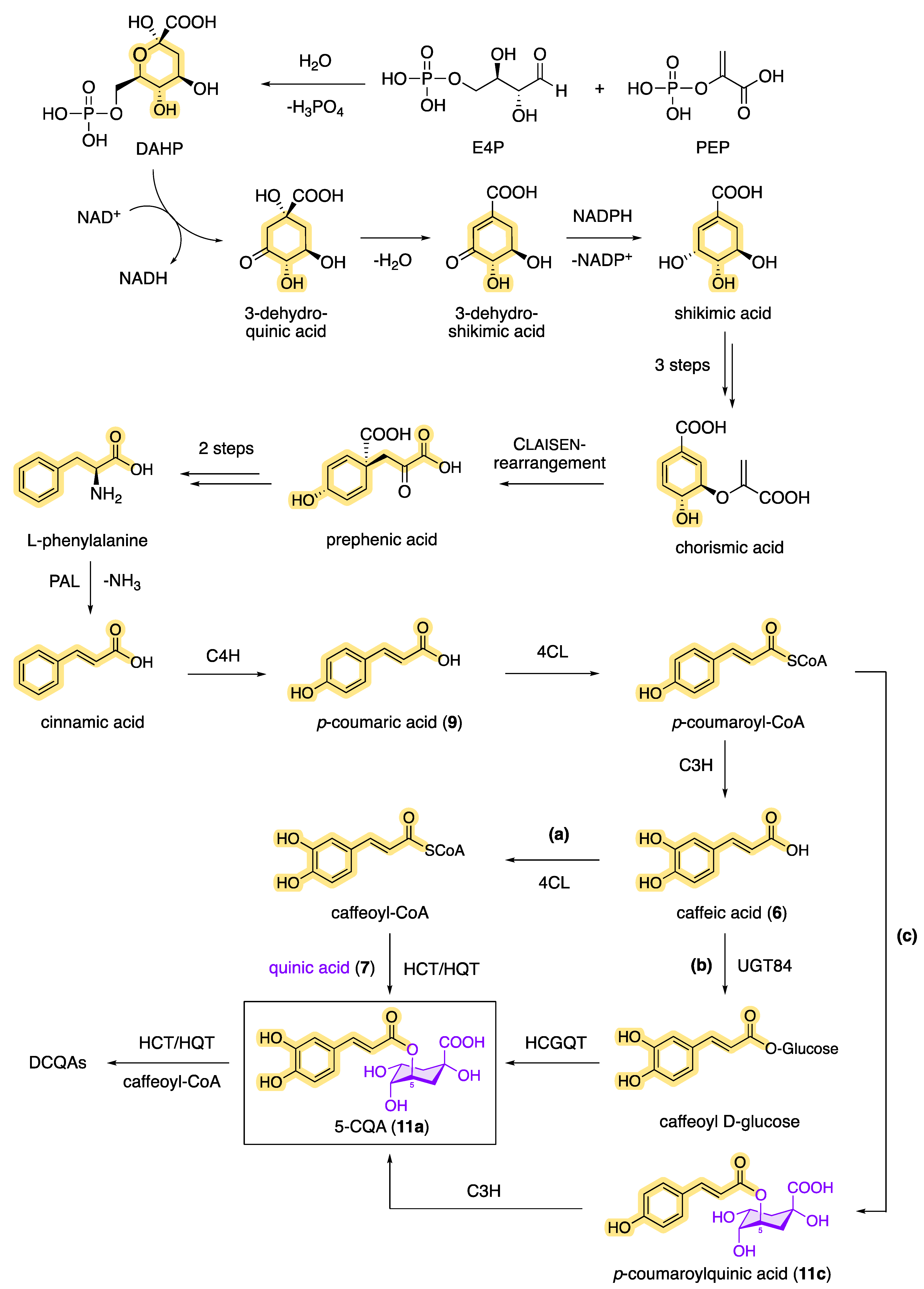
Plants can biosynthesize chlorogenic acids through a combination of the shikimic acid pathway and the phenylpropanoid pathway (Figure 8), with CQAs being an important intermediate in the biosynthesis of lignin [16][81][90]. The shikimic acid pathway starts with the enzyme-catalyzed cyclization of phosphoenolpyruvate (PEP, from glycolysis) and erythrose 4-phosphate (E4P, from the pentose phosphate pathway), in which 3-dehydroquinic acid is formed via 3-deoxyarabinoheptulosanate-7-phosphate (DAHP). Following the dehydratase-catalyzed elimination of water, 3-dehydroshikimic acid is then formed, which reacts by redox reaction under the influence of NADPH to form shikimic acid. The key intermediate, shikimic acid, is then converted into chorismic acid by ATP-dependent kinase-catalyzed phosphorylation and subsequent phosphate elimination. The cyclohexadiene prephenic acid is then formed by the enzymatically catalyzed Claisen rearrangement, which, after decarboxylation and subsequent transamination, results in the product of the shikimic pathway, L-phenylalanine. L-phenylalanine is the starting point of the phenylpropanoid pathway [91]. In the first step, ammonia is split off via phenylalanine ammonia lyase (PAL), resulting in cinnamic acid as a reaction product. Then, via p-coumaric acid, cinnamate 4-hydroxylase (C4H) and 4-hydroxycinnamoyl-CoA ligase (4CL) generate caffeic acid as another important key intermediate in the biosynthetic pathway of CQAs. Starting from caffeic acid (6), for example, 5-CQA (11a) can be formed in two different ways: (a) by 4CL via caffeoyl-CoA with a subsequent transferase-catalyzed (HCT/HQT) reaction and (b) by UGT84 via caffeoyl glucoside with a subsequent hydroxycinnamoyl D-glucose: quinate hydroxycinnamoyl transferase (HCGQT)-catalyzed reaction [92][93]. Also known is the synthesis of 5-CQA by (c) the enzymatic reaction of p-coumaric acid (9) to p-coumaroyl-CoA and the subsequent formation of p-coumaroylquinic acid. A subsequent reaction with the enzyme coumarate 3-hydroxylase (C3H) then allows biosynthetic access to 5-CQA (11a) [94]. How plants produce DCQAs or even TCQAs biosynthetically has not yet been fully elucidated, but it is likely that the acylation of monocaffeoylquinic acids with caffeoyl-CoA is catalyzed by HCT/HQT, which has already been demonstrated in tomatoes and sweet potatoes [95][96].
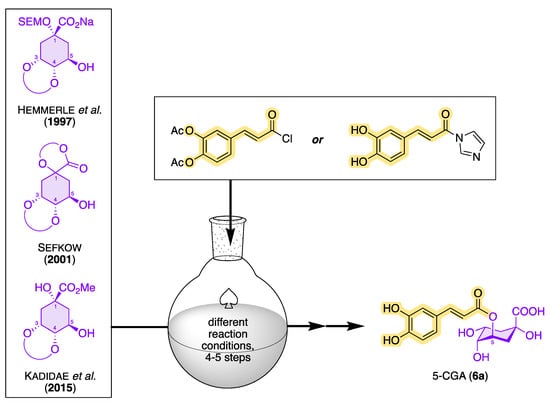
However, since plants always contain mixtures of many secondary metabolites, the isolation of CQAs from the corresponding plant extracts involves a great deal of effort. When extracting CQAs from plant material, chlorogenic acids can undergo various degradation reactions and/or isomerization under the influence of temperature, pH, and light, so the original CQA ratio can be strongly influenced and even falsified [97]. In addition to the storage conditions of plant materials, problems are also caused by thermal process steps that have an impact on the amount and ratio of chlorogenic acids. As mentioned before, the proportion of chlorogenic acids in thermally treated, and thus roasted, coffee is significantly reduced compared with green coffee [98]. Chemical conversion processes such as epimerization, acyl migration, and dehydration are responsible for this [99]. In order to adequately investigate the diverse biological activities of CQAs, chemical syntheses for the preparation of larger amounts of CQAs in pure form have come into focus. To date, there are only a few totally synthetic strategies starting from differently protected quinic acid and caffeic acid derivatives with moderate to good yields. An overview of possible synthesis strategies for 5-CQA (11a) starting from quinic acid acetals is shown in Figure 9 [100][101][102]. In 1955, Panizzi et al. reported the first synthesis of 5-CQA (11a). However, the synthesis from quinic acid was only possible in seven steps, with a low yield of <5% [103][104]. The choice of the protecting group and its removal as quantitatively as possible in the final step of the synthesis proved to be a major challenge. During the synthesis, it is important to keep in mind that deprotection under basic conditions should be avoided, since CQAs have been shown to be sensitive to oxidation reactions. Synthetic strategies should therefore exclusively rely on protecting groups that can be removed under acidic conditions. Hemmerle et al. were able to produce 5-CQA in a 5-step synthesis with an overall yield of 20–32%, starting from a quinic acid acetal and using acid-labile-protecting groups [91]. Sefkow was the first to be able to increase the yield in the synthesis of 5-CQA in 2001 to 65%, when he presented a 4-step synthesis strategy starting from quinic acid (7) that proceeded via a fully silyl-protected isolable intermediate and that also used acid-labile-protecting groups [92]. Fourteen years later, Kadidae et al. reported a short total synthesis of 5-CQA, but this synthetic approach was not quite as successful (with 35% over four steps) as that of Sefkow [93]. From the point of view of sustainability, the synthesis route of Hemmerle et al. was the best, as predominantly inexpensive and stable reagents were used. To date, however, the synthesis of 5-CQA according to Sefkow’s synthetic protocol is the most promising route, with the fewest number of synthetic steps and the highest yield. Sefkow et al. also reported synthetic strategies for the synthesis of 1-CQA, 3-CQA, and 4-CQA via quinic acid acetals with differently protected caffeic acid derivatives [105]. In addition, synthesis routes for the preparation of isochlorogenic acids are also known in the literature; however, these synthesis routes have not yet been able to surpass the synthesis protocol of Sefkow, and they are only associated with low to moderate yields [106][107][108][109].
The synthesis of tri-O-caffeoylquinic acids (TCQAs) from acetal-protected quinic acid lactones by acylation has been successfully demonstrated several times using a variant of the Schotten–Baumann method and via Steglich esterification [81][110].
3. Amounts of CQA in Coffee By-Products
As already mentioned, chlorogenic acids are found in a variety of plants. In coffee, the level of CQAs can vary widely depending on the degree of roasting and the geographical origin. The CQA content in roasted coffee ranges from 0.4–3.4 g/100 g (C. arabica) and 2.1–6.4 g/100 g (C. canephora) [44][111][112][113], while the amount of 5-CQA in green coffee has been shown to be higher at 6.0–9.0 g/100 g [114][115]. Processing, especially roasting, modifies dramatically the phenolic composition of coffee, but creates the characteristic aroma, flavor, and color of coffee beverages. In comparison with coffee and Prunus spp. cherry juice (85 mg/L), parts of the potato plant (leaves, sprout, root material) and the uncooked potato tuber contain very little amounts of 5-CQA, about 0.02–0.75 g/100 g [116][117]. Table 1 shows the amounts of monocaffeoylquinic acids and dicaffeoylquinic acids (isochlorogenic acids A–C) from coffee by-products with reference to the literature. It is noticeable that the respective values for CQAs in all coffee by-products vary quite widely. This is due to a number of factors, such as species and variety, different post-harvest processing methods, degree of ripeness, extent of environmental conditions, agricultural practices, and region of origin. What is also striking is that 5-caffeoylquinic acid (11a) is the most frequently occurring monocaffeoylquinic acid in all coffee by-products in terms of quantity, and the amounts of 3-CQA and 4-CQA are comparatively low. For this reason, the total amount of polyphenolic compounds is often given in the literature, with 5-CQA making up the largest proportion. Based on the amount of 5-CQA, the following sequence of coffee by-products can be formulated according to the amounts in Table 1: coffee husks > coffee pulp > silver skin > spent ground coffee > green unroasted beans > coffee leaves > coffee flowers (blossoms) > parchment. From the dicaffeoylquinic acids, 3,5-DCQA (16a) is mostly found in coffee by-products, although not all coffee by-products have been shown to contain DCQA in the literature.
Table 1. Amounts of chlorogenic acids in coffee by-products.
| Coffee By-Product |
Chlorogenic Acid Content, Expressed in [g/100 g] (unless Otherwise Stated) * | Ref. | |||||
|---|---|---|---|---|---|---|---|
| 3-CQA (10a) |
4-CQA (12a) |
5-CQA (11a) |
3,4-DCQA (16d) |
3,5-DCQA (16a) |
4,5-DCQA (16c) |
||
| coffee flowers (blossoms) | n.r. | n.r. | 0.13–2.64 | 0.01–0.25 | 0.19–5.84 | n.r. | [118] |
| 0.01–0.07 (in total) | [119] | ||||||
| coffee leaves | 0.04–0.30 | 0.09–0.84 | 1.39–5.58 | 0.03–0.18 | 0.01–1.13 | 0.01–0.12 | [120] |
| 1.1–18.0 mg/L | 4.2–30.0 mg/L | 1.0–190.0 mg/L ** | n.r. | n.r. | n.r. | [121] | |
| coffee pulp | n.r. | n.r. | 0.03–0.13 | n.r. | n.r. | n.r. | [122][123] |
| n.r. | n.r. | 2.3 | n.r. | n.r. | n.r. | [124] | |
| 0.03 | 0.04 | 0.22 | 0.05 | 0.06 | 0.03 | [125] | |
| n.r. | n.r. | 0.13–2.44 | n.r. | n.r. | n.r. | [126] | |
| n.r. | n.r. | 2.40 | n.r. | n.r. | n.r. | [127] | |
| coffee husk | n.r. | n.r. | 13.3 | n.r. | n.r. | n.r. | [124] |
| 0.01 | 0.02 | 0.57 | 0.08 | 0.15 | 0.04 | [125] | |
| n.r. | n.r. | 2.50 | n.r. | n.r. | n.r. | [127] | |
| n.r. | n.r. | 0.04–0.17 | n.r. | n.r. | n.r. | [128] | |
| n.r. | n.r. | 0.17 | n.r. | n.r. | n.r. | [129] | |
| n.r. | n.r. | 0.02–0.17 | n.r. | n.r. | n.r. | [128] | |
| n.r. | n.r. | 69.9 mg/L ** | n.r. | n.r. | n.r. | [130] | |
| silver skin | 1.80–5.16 | 2.38–6.02 | 7.33–8.98 | 0.93–1.04 | 0.55–1.00 | 0.79–1.06 | [131] |
| 0.15 | 0.09 | 0.20 | n.r. | n.r. | n.r. | [132] | |
| n.r. | n.r. | 2.2 | n.r. | n.r. | n.r. | [133] | |
| n.r. | n.r. | 0.94–2.13 | n.r. | n.r. | n.r. | [129] | |
| n.r. | n.r. | 0.39 | n.r. | n.r. | n.r. | [134] | |
| n.r. | n.r. | 20–30 mg/L ** | n.r. | n.r. | n.r. | [130] | |
| n.r. | n.r. | 0.02–0.04 | n.r. | n.r. | n.r. | [134] | |
| n.r. | n.r. | 3.00 | n.r. | n.r. | n.r. | [127] | |
| parchment | n.r. | n.r. | 0.61 | n.r. | n.r. | n.r. | [129] |
| n.r. | n.r. | 0.40 | n.r. | n.r. | n.r. | [135] | |
| spent coffee grounds | 1.50 | 1.62 | 1.87 | 0.29 | 1.00 | 1.70 | [131] |
| n.r. | n.r. | 2.30 | n.r. | n.r. | n.r. | [127] | |
| 0.62–1.32 (in total) | 3.31–5.79 (in total) | [136] | |||||
| green unroasted beans | 1.25 | 1.94 | 13.4 | 0.66 | 1.10 | 0.63 | [131] |
| n.r. | n.r. | 3.6–4.4 | n.r. | n.r. | n.r. | [135] | |
* n.r. = not reported; ** data reported for a beverage prepared from the material. For standardization and for a better comparison of the values among each other, the values found in the literature were converted into the unit [g/100 g].
Wirz et al. determined a maximum content of 5-CQA in coffee flowers (blossoms) of 2.64 g/100 g, whereas the amounts of 3-CQA and 5-CQA were negligibly small or little investigated. A concentration of up to 5.84 g/100 g 3,5-DCQA and only 0.25 g/100 g 3,4-DCQA was found in coffee flowers. The concentration of 3,5-DCQA is about twice as high as the concentration of 5-CQA. A similarly high concentration of up to 5.79 g/100 g DCQAs was also found in spent coffee grounds. Even if the information relates to the total amount of all dicaffeoylquinic acids, it can be assumed that 3,5-DCQA (isochlorogenic acid A) is the main isochlorogenic acid in terms of quantity. Using HPLC, a concentration of up to 5.58 g/100 g 5-caffeoylquinic acid could be determined from coffee leaves [118]. The content of 3-CQA and 4-CQA and especially the concentration of isochlorogenic acids were extremely low in comparison. Rodriguez-Gomez et al. determined the concentration of 5-CQA in coffee leaves using LC-EC and LC-qToF-MS to be 1.0–190 mg/L [121], with the different concentrations often varying greatly for reasons already explained. At 0.13–2.44 g/100 g, 5-CQA is also the monocaffeoylquinic acid in coffee pulp with the highest concentration. According to Esquivel et al., 3,4- and 3,5-DCQA are represented 2 to 40 times less abundantly than 5-CQA at 0.06 g/100 g [125]. In contrast, in coffee husks, Palomino Garcia et al. found a 5-CQA concentration of 13.3 g/100 g. A similarly high concentration of 5-CQA (13.4 g/100 g) was found by Regazzoni et al. in green unroasted coffee beans using HPLC-UV for quantification.
In silver skin, the concentration of 5-caffeoylquinic acid (5-CQA) determined from the literature is between 0.02 (minimum) and 9.0 g/100 g (maximum). Only Regazzoni and co-workers were able to extract and quantify the other mono- and dicaffeoylquinic acids by liquid chromatography [131]. In comparison with the other coffee by-products, the determined concentrations of 3-CQA (up to 5.16 g/100 g) and 4-CQA (up to 6.02 g/100 g) come very close to the concentration of the main chlorogenic acid 5-CQA (up to 8.89 g/100 g), while in other by-products 3-CQA and the 4-CQA occur in low concentrations compared with 5-CQA. The concentration of dicaffeoylquinic acids in silver skin, on the other hand, is largely equally distributed among the three DCQAs in a ratio of about 1:1:1 (each with up to 1.00 g/100 g) [131]. On the contrary, the concentration of 5-CQA in the coffee by-products parchment and spent coffee grounds is quite low at 0.4–0.61 g/100 g and 1.32–2.30 g/100 g, respectively [127][129][131][136]. However, it is interesting that the monocaffeoylquinic acids 3-CQA, 4-CQA, and 5-CQA in spent coffee grounds occur in a ratio of about 1:1.1:1.3 and differ less in terms of quantity than in other coffee by-products [131].
According to Murthy and Naidu, coffee by-products in total contain about 2.3–3.0% chlorogenic acids as the phenolic compound [137]. To verify this statement, the concentrations from the literature (from Table 1) for each coffee by-product were listed using the unit g/100 g, which allows the direct conversion into a percentage (%). Table 2 shows the results. The literature concentrations result in a concentration range of 0.8–5.5% (min–max) chlorogenic acids, with a total average value of 3.1%. The determined range is a bit wider than given by the authors, but the calculated total average value fits very well.
Table 2. Amounts of 5-caffeoylquinic acid in coffee by-products.
| Coffee By-Product | Min (%) | Max (%) | Average (%) |
|---|---|---|---|
| coffee flowers (blossoms) | 0.01 | 2.60 | 1.31 |
| coffee leaves | 1.39 | 5.60 | 3.50 |
| coffee pulp | 0.03 | 2.40 | 1.22 |
| coffee husk | 0.02 | 13.3 | 6.66 |
| cascara | 0.01 | 0.01 | 0.01 |
| silver skin | 0.02 | 8.98 | 4.50 |
| parchment | 0.40 | 0.61 | 0.51 |
| spent coffee grounds | 1.32 | 2.30 | 1.81 |
| green unroasted beans | 3.60 | 13.4 | 8.50 |
| total | 0.76 | 5.47 | 3.11 |
For comparison, Belitz et al. reported that green coffee from C. arabica contains 3.0–5.6% and C. canephora 4.4–6.6% chlorogenic acid (based on dry matter), while the chlorogenic acid content in roasted coffee is 2.7% for C. arabica and 3.1% for C. canephora, based on the dry matter [138]. Perrone et al. describe a chlorogenic acid content of 4–12% in the raw coffee components in the mass [139], which can be attributed to the transformation into related compounds (see Figure 6).
This entry is adapted from the peer-reviewed paper 10.3390/molecules28145540
References
- Lachenmeier, D.W.; Teipel, J.; Scharinger, A.; Kuballa, T.; Walch, S.G.; Grosch, F.; Brunzel, M.; Okaru, A.O.; Schwarz, S. Fully automated identification of coffee species and simultaneous quantification of furfuryl alcohol using NMR spectroscopy. J. AOAC Int. 2020, 103, 306–314.
- Rimbach, G.; Nagursky, J.; Erbersdobler, H.F. Lebensmittel-Warenkunde für Einsteiger, 2nd ed.; Springer: Berlin, Germany, 2015.
- Rösemann, U. Coffea—Für viele müde Menschen die erste Pflanze des Tages. Biol. Unserer Zeit 2017, 47, 336–337.
- Tritsch, N.; Steger, M.C.; Segatz, V.; Blumenthal, P.; Rigling, M.; Schwarz, S.; Zhang, Y.; Franke, H.; Lachenmeier, D.W. Risk Assessment of Caffeine and Epigallocatechin Gallate in Coffee Leaf Tea. Foods 2022, 11, 263.
- Hegnauer, R. Chemotaxonomie der Pflanzen: Eine Übersicht über die Verbreitung und die Systematische Bedeutung der Pflanzenstoffe; Band 21; Birkhäuser: Basel, Switerland, 1973; p. 169.
- Baltes, W. Lebensmittelchemie; Springer: Berlin/Heidelberg, Germany, 2007; pp. 398–413.
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495.
- Lai, W.T.; Khong, N.M.H.; Lim, S.S.; Hee, Y.Y.; Sim, B.I.; Lau, K.Y. A review: Modified agricultural by-products for the development and fortification of food products and nutraceuticals. Trends Food Sci. Technol. 2017, 59, 148–160.
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural waste peels as versatile biomass for water purification—A review. Chem. Eng. J. 2015, 270, 244–271.
- Klingel, T.; Kremer, J.I.; Gottsein, V.; Rajcic de Rezende, T.; Schwarz, S.; Lachenmeier, D.W. A Review of coffee by-products including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 665.
- European Union. European Union Regulation (EU) 2015/2283 of the European parliament and of the council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Off. J. Eur. Union 2015, L327, 1–22.
- EFSA. Novel Food. Available online: http://www.efsa.europa.eu/en/topics/topic/novel-food (accessed on 27 September 2022).
- Blinová, L.; Sirotiak, M.; Pastierova, A.; Soldán, M. Review: Utilization of Waste from Coffee Production. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2017, 25, 91–101.
- Lachenmeier, D.W.; Schwarz, S.; Rieke-Zapp, J.; Cantergiani, E.; Rawel, H.; Martín-Cabrejas, M.A.; Martuscelli, M.; Gottstein, V.; Angeloni, S. Coffee By-Products as Sustainable Novel Foods: Report of the 2nd International Electronic Conference on Foods—“Future Foods and Food Technologies for a Sustainable World”. Foods 2021, 11, 3.
- Lachenmeier, D.W.; Rajcic de Rezende, T.; Schwarz, S. An Update on sustainable valorization of coffee by-products as novel foods within the European Union. Biol. Life Sci. Forum 2021, 6, 37.
- Payen, S. Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences; Bachelier: Paris, France, 1846; Volume 23, pp. 244–251.
- Payen, S. Untersuchung des Kaffees. Ann. Chem. Pharm. 1846, 60, 286–294.
- Rochleder, F. Untersuchung der Kaffeebohnen. Justus Liebigs Ann. Chem. 1846, 59, 300–310.
- Gorter, K. Beiträge zur Kenntnis des Kaffees (Erste Abhandlung). Justus Liebigs Ann. Chem. 1908, 358, 327–348.
- Freudenberg, K. Über Gerbstoffe. III. Chlorogensäure, der gerbstoff-artige Bestandteil der Kaffeebohnen. Ber. Dtsch. Chem. Ges. A 1920, 53, 232–239.
- Clifford, M.N.; Johnston, K.L.; Knigh, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911.
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421.
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant. Physiol. 2006, 18, 23–36.
- Cartwright, R.A.; Roberts, E.A.H.; Flood, E.A. Chem. Indust. 1955, 1062–1063.
- Corse, J.; Sondheimer, E.; Lundin, R. 3-feruloylquinic acid: A 3′-methyl ether of chlorogenic acid. Tetrahedron 1962, 18, 2107–2108.
- Awwad, S.; Issa, R.; Alnsour, L.; Albals, D.; Al-Momani, I. Quantification of Caffeine and Chlorogenic acid in Green and Roasted Coffee Samples Using HPLC-DAD and Evaluation of the Effect of Degree of Roasting on Their Levels. Molecules 2021, 26, 7502.
- Silva, N.; Mazzafera, P.; Cesarino, I. Should I stay or should I go: Are chlorogenic acids mobilized towards lignin biosynthesis? Phytochemistry 2019, 166, 112063.
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Ann. Rev. Plant. Biol. 2003, 54, 519–546.
- Fischer, H.O.L.; Dangschat, G. Konstitution der Chlorogensäure (3. Mitteil. Über Chinasäure Derivate). Ber. Dtsch. Chem. Ges. A 1932, 65, 1037–1040.
- Narita, Y.; Inouye, K. Chapter 21—Chlorogenic acids from coffee. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 189–199.
- Clifford, M.N. Researchgate. 2017. Available online: https://www.researchgate.net/publication/312591202 (accessed on 10 January 2023).
- IUPAC. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC) and IUPAC-IUB Commission on Biochemical Nomenclature (CBN). Nomenclature of cyclitols. Recommendations (1973). Biochem. J. 1976, 153, 23–31.
- Abrankó, L.; Clifford, M.N. An Unambiguous Nomenclature for the Acyl-quinic Acids Commonly known as Chlorogenic Acids. J. Agric. Food Chem. 2017, 65, 3602–3608.
- Corse, J.; Patterson, D.C. 3-O-Sinapolyquinic acid. Phytochemistry 1969, 8, 203–205.
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158.
- Parejo, I.; Jauregui, O.; Sánchez-Rabaneda, F.; Viladomat, F.; Bastida, J.; Codina, C. Separation and characterization of phenolic compounds in fennel (Foeniculum vulgare) using liquid chromatography–negative electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2004, 52, 3679–3687.
- Clifford, M.N.; Kellard, B.; Birch, G.G. Characterisation of chlorogenic acids by simultaneous isomerisation and transesterification with tetramethylammonium hydroxide. Food Chem. 1989, 33, 115–123.
- Clifford, M.N.; Kellard, B.; Birch, G.G. Characterisation of caffeoylferuloylquinic acids by simultaneous isomerisation and transesterification with tetramethylammonium hydroxide. Food Chem. 1989, 34, 81–88.
- Dawidowicz, A.L.; Typek, R. Transformation of chlorogenic acids during the coffee beans roasting process. Eur. Food Res. Technol. 2017, 243, 379–390.
- Macheiner, L.; Schmidt, A.; Schreiner, M.; Mayer, H.K. Green coffee infusion as a source of caffeine and chlorogenic acid. J. Food Compos. Anal. 2019, 84, 103307.
- Liang, N.; Xue, W.; Kennepohl, P.; Kitts, D.D. Interaction between major chlorogenic acid isomers and chemical changes in coffee brew that affect antioxidant activities. Food Chem. 2016, 213, 251–259.
- Bobková, A.; Jakabová, S.; Belej, L.; Jurcaga, L.; Capla, J.; Bobko, M.; Demianová, A. Analysis of caffeine and chlorogenic acids content regarding the preparation method of coffee beverage. Int. J. Food. Eng. 2021, 17, 403–410.
- Ludwig, I.A.; Mena, P.; Calani, L.; Concepción, C.; Del Rio, D.; Lean, M.E.J.; Crozier, A. Variations in caffeine and chlorogenic contents of coffee: What are we drinking? Food Funct. 2014, 5, 1718.
- Farah, A.; De Paulis, T.; Moreira, D.P.; Trugo, L.C.; Martin, P.R. Chlorogenic acids and lactones in regular and water-decaffeinated arabica coffees. J. Agric. Food Chem. 2006, 54, 374–381.
- Jiang, D.; Peterson, D.G. Role of hydroxycinnamic acids in food flavor: A brief overview. Phytochem. Rev. 2010, 9, 187–193.
- Kuhnert, N. Analysis of chlorogenic acid lactones and caffeoyl shikimic acids in roasted coffee. Eng. Food Sci. 2013, 17, 568–576.
- Duke, J.A. List of Plants for Chlorogenic Acid; Dr. Duke’s Phytochemical and Ethnobotanical Database; U.S. Department of Agriculture: Washington, DC, USA, 1992.
- Daraee, A.; Ghoreishi, S.M.; Hedayati, A. Supercritical CO2 extraction of chlorogenic acid from sunflower (Helianthus annuus) seed kernels: Modeling and optimization by response surface methodology. J. Supercrit. Fluids 2019, 144, 19–27.
- Kweon, M.-H.; Hwang, H.-J.; Sung, H.-C. Identification and Antioxidant Activity of Novel Chlorogenic Acid Derivatives from Bamboo (Phyllostachys edulis). J. Agri. Food Chem. 2001, 49, 4646–4652.
- Jalal, M.A.F.; Read, D.J.; Haslam, E. Phenolic composition and its seasonal variation in Calluna vulgaris. Phytochemistry 1982, 21, 1397–1401.
- Petrisor, G.; Motelica, L.; Craciun, L.N.; Oprea, O.C.; Ficai, D.; Ficai, A. Melissa officinalis: Composition, Pharmacological Effects and Derived Release Systems—A Review. Int. J. Mol. Sci. 2022, 23, 3591.
- Von Bruchhausen, F. Hagers Handbuch der Pharmazeutischen Praxis; Springer: Berlin/Heidelberg, Germany, 1949; ISBN 3-540-52688-9.
- Leung, A.Y.; Foster, S. Encyclopedia of Common Natural Ingredients, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1995; p. 649.
- Kim, S.M.; Shang, Y.F.; Um, B.H. Preparative separation of chlorogenic acid by centrifugal partition chromatography from highbush blueberry leaves (Vaccinium corymbosum L.). Phytochem. Anal. 2010, 21, 457–462.
- Mei, Y.; Sun, H.; Du, G.; Wang, X.; Lyu, D. Exogenous chlorogenic acid alleviates oxidative stress in apple leaves by enhancing antioxidant capacity. Sci. Hortic. 2020, 274, 109676.
- Kliewer, W.M. Sugars and Organic Acids of Vitis vinifera. Plant Physiol. 1966, 41, 923–931.
- Luthria, D.L.; Mukhopadhyay, S. Influence of Sample Preparation on Assay of Phenolic Acids from Eggplant. J. Agric. Food Chem. 2006, 54, 41–47.
- Cheng, G.W.; Crisosto, C.H. Browning Potential, Phenolic Composition, and Polyphenoloxidase Activity of Buffer Extracts of Peach and Nectarine Skin Tissue. J. Am. Soc. Hortic. Sci. 1995, 120, 835–838.
- Stacewicz-Sapuntzakis, M.; Bowen, P.E.; Hussain, E.A.; Damayanti-Wood, B.I.; Farnsworth, N.R. Chemical composition and potential health effects of prunes: A functional food? Crit. Rev. Food Sci. Nutr. 2001, 41, 251–286.
- Clifford, M.N.; Shutler, S.; Thomas, G.A.; Ohiokpehai, O. The chlorogenic acids content of coffee substitutes. Food Chem. 1987, 24, 99–107.
- Zhen, J.; Villani, T.S.; Guo, Y.; Qi, Y.; Chin, K.; Pan, M.-H.; Ho, C.-T.; Simon, J.E.; Wu, Q. Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem. 2016, 190, 673–680.
- Gutiérrez Ortiz, A.L.; Berti, F.; Navarini, L.; Crisafulli, P.; Colomban, S.; Forzato, C. Aqueous extracts of walnut (Juglans regia L.) leaves: Quantitative analyses of hydroxycinnamic and chlorogenic acids. J. Chromatogr. Sci. 2018, 56, 753–760.
- Mijangos-Ramos, I.F.; Zapata-Estrella, H.E.; Ruiz-Vargas, J.A.; Escalante-Erosa, F.; Gómez-Ojeda, N.; García-Sosa, K.; Cechinel-Filho, V.; Meira-Quintão, N.L.; Peña-Rodríguez, L.M. Bioactive dicaffeoylquinic acid derivatives from the root extract of Calea urticifolia. Rev. Bras. Farmacogn. 2018, 28, 339–343.
- Yin, X.-L.; Xu, B.-Q.; Zhang, Y.-Q. Gynura divaricata rich in 3, 5-/4, 5-dicaffeoylquinic acid and chlorogenic acid reduces islet cell apoptosis and improves pancreatic function in type 2 diabetic mice. Nutr. Metab. 2018, 15, 73.
- Lee, Y.-J.; Jang, Y.-N.; Han, Y.-M.; Kim, H.-M.; Jeong, J.-M.; Son, M.J.; Jin, C.B.; Kim, H.J.; Seo, H.S. Caffeoylquinic acid-rich extract of Aster glehni F. Schmidt ameliorates nonalcoholic fatty liver through the regulation of PPARδ and adiponectin in ApoE KO mice. PPAR Res. 2017, 2017, 3912567.
- Topal, M.; Gocer, H.; Topal, F.; Kalin, P.; Köse, L.P.; Gülçin, İ.; Çakmak, K.C.; Küçük, M.; Durmaz, L.; Gören, A.C.; et al. Antioxidant, antiradical, and anticholinergic properties of cynarin purified from the Illyrian thistle (Onopordum illyricum L.). J. Enzym. Inhib. Med. Chem. 2016, 31, 266–275.
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; da Silveira, T.F.F.; Filho, J.T.; Godoy, H.T. Chlorogenic acid isomer contents in 100 plants commercialized in Brazil. Food Res. Int. 2017, 99, 522–530.
- Li, X.; Li, K.; Xie, H.; Xie, Y.; Li, Y.; Zhao, X.; Jiang, X.; Chen, D. Antioxidant and Cytoprotective Effects of the Di-O- Caffeoylquinic Acid Family: The Mechanism, Structure−Activity Relationship, and Conformational Effect. Molecules 2018, 23, 222.
- Mossi, A.; Echeverrigaray, S. Identification and characterization of antimicrobial components in leaf extracts of globe artichoke (Cynara scolymus L.). Acta Hortic. 1999, 501, 111–114.
- Mahmood, N.; Moore, P.; De Tommasi, N.; De Simone, F.; Colman, S.; Hay, A.; Pizza, C. Inhibition of HIV infection by caffeoylquinic acid derivatives. Antivir. Chem. Chemother. 1993, 4, 235–240.
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of Chlorogenic Acid in Regulating Glucose and Lipid Metabolism: A Review. Evid. Based Complement. Alternat. Med. 2013, 2013, 801457.
- Hur, J.Y.; Soh, Y.; Kim, B.-H.; Suk, K.; Sohn, N.W.; Kim, H.C.; Kwon, H.C.; Lee, K.R.; Kim, S.Y. Neuroprotective and neurotrophic effects of quinic acids from Aster scaber in PC12 cells. Biol. Pharm. Bull. 2001, 24, 921–924.
- Kim, S.S.; Park, R.Y.; Jeon, H.J.; Kwon, Y.S.; Chun, W. Neuroprotective effects of 3,5-dicaffeoylquinic acid on hydrogen peroxide-induced cell death in SH-SY5Y cells. Phytother. Res. 2005, 19, 243–245.
- Dos Santos, M.D.; Chen, G.; Almeida, M.C.; Soares, D.M.; de Souza, G.E.P.; Lopes, N.P.; Lantz, R.C. Effects of caffeoylquinic acid derivatives and C-flavonoid from Lychnophora ericoides on in vitro inflammatory mediator production. Nat. Prod. Commun. 2010, 5, 733–740.
- Islam, M.S.; Yoshimoto, M.; Yahara, S.; Okuno, S.; Ishiguro, K.; Yamakawa, O. Identification and characterization of foliar polyphenolic composition in sweet potato (Ipomoea batatas L.) genotypes. J. Agric. Food Chem. 2002, 50, 3718–3722.
- Yoshimoto, M.; Yahara, S.; Okuno, S.; Islam, M.S.; Ishiguro, K.; Yamakawa, O. Antimutagenicity of mono-, di-, and tricaffeoylquinic acid derivatives isolated from sweet potato (Ipomoea batatas L.) leaf. Biosci. Biotechnol. Biochem. 2002, 66, 2336–2341.
- Li, L.; Su, C.; Chen, X.; Wang, Q.; Jiao, W.; Luo, H.; Tang, J.; Wang, W.; Li, S.; Guo, S. Chlorogenic acids in cardiovascular disease: A review of dietary consumption, pharmacology, and pharmacokinetics. J. Agric. Food Chem. 2020, 68, 6464–6484.
- Zhao, Y.; Wang, J.; Ballevre, O.; Luo, H.; Zhang, W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Res. 2012, 35, 370–374.
- Wang, L.-N.; Wang, W.; Hattori, M.; Daneshtalab, M.; Ma, C.-M. Synthesis, Anti-HCV, Antioxidant and Reduction of Intracellular Reactive Oxygen Species Generation of a Chlorogenic Acid Analogue with an Amide Bond Replacing the Ester Bond. Molecules 2016, 21, 737.
- Ma, C.-M.; Kully, M.; Khan, J.K.; Hattori, M.; Daneshtalab, M. Synthesis of chlorogenic acid derivatives with promising antifungal activity. Bioorg. Med. Chem. 2007, 15, 6830–6833.
- Timmermann, B.N.; Hoffmann, J.J.; Jolad, S.D.; Schram, K.H.; Klenck, R.E.; Bates, R.B. Constituents of Chrysothamnus paniculatus 3: 3,4,5-tricaffeoylquinic acid (a new shikimate prearomatic) and 3,4-, 3,5-and 4,5-dicaffeoylquinic acids. J. Nat. Prod. 1983, 46, 365–368.
- Agata, I.; Goto, S.; Hatano, T.; Nishibe, S.; Okuda, T. 1,3,5-Tri-O-caffeoylquinic acid from Xanthium strumarium. Phytochemistry 1993, 33, 508–509.
- Merfort, I. Caffeoylquinic acids from flowers of Arnica montana and Arnica chamissonis. Phytochemistry 1992, 31, 2111–2113.
- Liu, L.; Zhang, J.; Zheng, B.; Guan, Y.; Wang, L.; Chen, L.; Cai, W. Rapid characterization of chlorogenic acids in Duhaldea nervosa based on ultra-high-performance liquid chromatography-linear trap quadrupole-Orbitrap-mess spectroscopy and mass spectral trees similarity filter technique. J. Sep. Sci. 2018, 41, 1764–1774.
- Kim, S.M.; Jeon, J.S.; Kang, S.W.; Jung, Y.J.; Ly, L.N.; Um, B.H. Content of antioxidative caffeoylquinic acid derivatives in field-grown Ligularia fischeri (Ledeb.) Turcz and responses to sunlight. J. Agric. Food Chem. 2012, 60, 5597–5603.
- Matsui, T.; Ebuchi, S.; Fujise, T.; Abesundara, K.J.; Doi, S.; Yamada, H.; Matsumoto, K. Strong antihyperglycemic effects of water-soluble fraction of Brazilian propolis and its bioactive constituent, 3,4,5-tri-O-caffeoylquinic acid. Biol. Pharm. Bull. 2004, 27, 1797–1803.
- Scholz, E.; Heinrich, M.; Hunkler, D. Caffeoylquinic acids and some biological activities of Pluchea symphytifolia. Planta Med. 1994, 60, 360–364.
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516.
- Smith, P.; Ho, C.K.; Takagi, Y.; Djaballah, H.; Shuman, S. Nanomolar Inhibitors of Trypanosoma brucei RNA Triphosphatase. mBio 2016, 7, e0058-16.
- Comino, C.; Hehn, A.; Moglia, A.; Menin, B.; Bourgaud, F.; Lanteri, S.; Portis, E. The isolation and mapping of a novel hydroxycinnamoyltransferase in the globe artichoke chlorogenic acid pathway. BMC Plant Biol. 2009, 9, 30.
- Zhang, J.; Yang, Y.; Zheng, K.; Xie, M.; Feng, K.; Jawdy, S.S.; Gunter, L.E.; Ranjan, P.; Singan, V.R.; Engle, N.; et al. Genome-wide association studies and expression-based quantitative trait loci analyses reveal roles of HCT2 in caffeoylquinic acid biosynthesis and its regulation by defense-responsive transcription factors in Populus. New Phytol. 2018, 220, 502–516.
- Menin, B.; Comino, C.; Moglia, A.; Dolzhenko, Y.; Portis, E.; Lanteri, S. Identification and mapping of genes related to caffeoylquinic acid synthesis in Cynara cardunculus L. Plant Sci. 2010, 179, 338–347.
- Moglia, A.; Acquadro, A.; Eljounaidi, K.; Milani, A.M.; Cagliero, C.; Rubiolo, P.; Genre, A.; Cankar, K.; Beekwilder, J.; Comino, C. Genome-wide identification of BAHD acyltransferases and in vivo characterization of HQT-like enzymes involved in caffeoylquinic acid synthesis in globe artichoke. Front. Plant Sci. 2016, 7, 1424.
- Villegas, R.; Kojima, M. Purification and characterization of hydroxycinnamoyl D-glucose. Quinate hydroxycinnamoyl transferase in the root of sweet potato, Ipomoea batatas Lam. J. Biol. Chem. 1986, 261, 8729–8733.
- Lallemand, L.A.; Zubieta, C.; Lee, S.G.; Wang, Y.; Acajjaoui, S.; Timmins, J.; McSweeney, S.; Jez, J.M.; McCarthy, J.G.; McCarthy, A.A. A structural basis for the biosynthesis of the major chlorogenic acids found in coffee. Plant Physiol. 2012, 160, 249–260.
- Moglia, A.; Lanteri, S.; Comino, C.; Hill, L.; Knevitt, D.; Cagliero, C.; Rubiolo, P.; Bornemann, S.; Martin, C. Dual catalytic activity of hydroxycinnamoyl-Coenzyme A quinate transferase from tomato allows it to moonlight in the synthesis of both mono-and dicaffeoylquinic acids. Plant Physiol. 2014, 166, 1777–1787.
- Xue, M.; Shi, H.; Zhang, J.; Liu, Q.-Q.; Guan, J.; Zhang, J.-Y.; Ma, Q. Stability and Degradation of Caffeoylquinic acids under different storage conditions studied by high-performance liquid chromatography with Photo Diode Array Detection and High-performance liquid chromatography with electrospray ionization collision-induced dissociation tandem mass spectrometry. Molecules 2016, 21, 948.
- Kaiser, N.; Birkholz, D.; Colomban, S.; Navarini, L.; Engelhardt, U.H. A New Method for the preparative Isolation of chlorogenic acid lactones from coffee and model roasts of 5-caffeoylquinic acid. J. Agric. Food Chem. 2013, 61, 6937–6941.
- Jaiswal, R.; Matei, M.F.; Golon, A.; Witt, M.; Kuhnert, N. Understanding the fate of chlorogenic acids in coffee roasting using mass spectrometry based targeted and non-targeted analytical strategies. Food Funct. 2012, 3, 976–984.
- Hemmerle, H.; Burger, H.-J.; Below, P.; Schubert, G.; Rippel, R.; Schindler, P.W.; Paulus, E.; Herling, A.W. Chlorogenic acid and synthetic chlorogenic acid derivatives: Novel inhibitors of hepatic glucose-6-phosphate translocase. J. Med. Chem. 1997, 40, 137–145.
- Sefkow, M. First efficient synthesis of chlorogenic acid. Eur. J. Org. Chem. 2001, 2001, 1137–1141.
- Kadidae, L.O.; Usami, A.; Koyama, T.; Honda, M.; Kunimoto, K.-K. New route for synthesis of 3- and 5-caffeoylquinic acids via protected quinic acids. Eur. J. Chem. 2015, 6, 367–373.
- Panizzi, L.; Scarpati, M.L.; Oriente, G. Sintesi dell’acido clorogenico. Experientia 1955, 11, 383–384.
- Panizzi, L.; Scarpati, M.L.; Oriente, G. Synthesis of Chlorogenic acid. Gazz. Chim. Ital. 1956, 86, 913–922.
- Sefkow, M.; Kelling, A.; Schilde, U. First Efficient Syntheses of 1-, 4-, and 5-Caffeoylquinic Acid. Eur. J. Org. Chem. 2001, 2001, 2735–2742.
- Oyama, K.; Watanabe, N.; Yamada, T.; Suzuki, M.; Sekiguchi, Y.; Kondo, T.; Yoshida, K. Efficient and versatile synthesis of 5-O-acylquinic acids with a direct esterification using a p-methoxybenzyl quinate as a key intermediate. Tetrahedron 2015, 71, 3120–3130.
- Nagaoka, T.; Banskota, A.H.; Xiong, Q.; Tezuka, Y.; Kadota, S. Synthesis and antihepatotoxic and antiproliferative activities of di-and tri-O-caffeoylquinic acid derivatives. J. Trad. Med. 2001, 18, 183–190.
- Miyamae, Y.; Kurisu, M.; Han, J.; Isoda, H.; Shigemori, H. Structure−activity relationship of caffeoylquinic acids on the accelerating activity on ATP production. Chem. Pharm. Bull. 2011, 59, 502–507.
- Raheem, K.S.; Botting, N.P.; Williamson, G.; Barron, D. Total synthesis of 3, 5-O-dicaffeoylquinic acid and its derivatives. Tetrahedron Lett. 2011, 52, 7175–7177.
- Brummond, K.M.; DeForrest, J.E. Synthesis of the Naturally Occurring (−)-1, 3, 5-Tri-O-Caffeoylquinic Acid. Synlett 2009, 2009, 1517–1519.
- Fujioka, K.; Shibamoto, T. Chlorogenic acid and caffeine contents in various commercial brewed coffees. Food Chem. 2008, 106, 217–221.
- Misto, M.; Lestari, N.P.; Purwandan, E. Chlorogenic acid content of local robusta coffee at variations of roasting temperature. J. Pendidik. Fis. Indon. 2022, 18, 25–32.
- Viencz, T.; Acre, L.B.; Rocha, R.B.; Alves, E.A.; Ramalho, A.R.; Benassi, M.T. Caffeine, trigonelline, chlorogenic acids, metlanoidins, and diterpenes contents of coffea canephora coffees produced in the amazon. J. Food. Comp. Anal. 2023, 117, 105140.
- Budavari, S.; Angelica, E. Caffeic Acid. Chlorogenic Acid. Coffee, Green. Crataegus. Maté. In The Merck Index, 12th ed.; Merck & Co., Inc.: Whitehall, NJ, USA, 1996; p. 109ff.
- Wasserman, G.; Stahl, H.D.; Rehman, W.; Whitman, P. Coffee. In Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; John Wiley and Sons: New York, NY, USA, 1993; Volume 6, pp. 793–811.
- Friedman, M. Chemistry, biochemistry, and dietary role of potato polyphenols. A review. J. Agric. Food Chem. 1997, 45, 1523–1540.
- Shahrzad, S.; Bitsch, I. Determination of some pharmacologically active phenolic acids in juice by high-performance liquid chromatography. J. Chromatogr. A 1996, 741, 223–231.
- Wirz, K.; Schwarz, S.; Richling, E.; Walch, S.G.; Lachenmeier, D.W. Coffee Flower as a Promising Novel Food—Chemical Characterization and Sensory Evaluation. Biol. Life Sci. Forum 2022, 18, 53.
- De Abreu Pinheiro, F.; Elias, L.F.; de Jesus Filho, M.; Modol, M.U.; de Cassia Gomas Rocha, J.; Lemos, M.F.; Scherer, R.; Carodos, W.S. Arabica and Conilon coffee flowers: Bioactive compounds and antioxidant capacity under different processes. Food Chem. 2021, 336, 127701.
- Monteiro, Â.; Colomban, S.; Azinheira, H.G.; Guerra-Guimarães, L.; Do Céu Silva, M.; Navarini, L.; Resmini, M. Dietary Antioxidants in Coffee Leaves: Impact of Botanical Origin and Maturity on Chlorogenic Acids and Xanthones. Antioxidants 2020, 9, 6.
- Rodriguez-Gomez, R.; Vanheuverzwjin, J.; Souard, F.; Delporte, C.; Stevingy, C.; Stoffelen, P.; De Braekeleer, K.; Kauffmann, J.-M. Determination of Three Main Chlorogenic Acids in Water Extracts of Coffee Leaves by Liquid Chromatography Coupled to an Electrochemical Detector. Antioxidants 2018, 7, 143.
- Torres-Mancera, M.T.; Baqueiro-Peña, I.; Figueroa-Montero, A.; Rodríguez-Serrano, G.; González-Zamora, E.; Favela-Torres, E.; Saucedo-Castañeda, G. Biotransformation and improved enzymatic extraction of chlorogenic acid from coffee pulp by filamentous fungi. Biotechnol. Prog. 2013, 29, 337–345.
- Torres-Mancera, M.T.; Cόrdova Loόpez, J.; Rodríguez Serrano, G.; Roussos, S.; Ramírez-Coronel, M.A.; Favela-Torres, E.; Saucedo-Castañeda, G. Enzymatic extraction of hydroxycinnamic acids from coffee pulp. Food Technol. Biotechnol. 2011, 49, 369–373.
- Palomino García, L.R.; Biasetto, C.R.; Araujo, A.R.; Del Bianchi, V.L. Enhanced extraction of phenolic compounds from coffee industry’s residues through solid state fermentation by Penicillium purpurogenum. Food Sci. Technol. 2015, 35, 704–711.
- Esquivel, P.; Viñas, M.; Steingass, C.B.; Gruschwitz, M.; Guevara, E.; Carle, R.; Schweiggert, R.M.; Jiménez, V.M. Coffee (Coffea arabica L.) by-Products as a Source of Carotenoids and Phenolic Compounds—Evaluation of Varieties with Different Peel Color. Front. Sustain. Food Syst. 2020, 4, 590–597.
- Rodríguez-Durán, L.V.; Ramírez-Coronel, M.A.; Aranda-Delgado, E.; Nampoothiri, K.M.; Favela-Torres, E.; Aguilar, C.N.; Saucedo-Castañeda, G. Soluble and Bound Hydroxycinnamates in Coffee Pulp (Coffea arabica) from Seven Cultivars at Three Ripening Stages. J. Agric. Food Chem. 2014, 62, 7869–7876.
- Murthy, P.S.; Madhava-Naidu, M. Sustainable Management of Coffee Industry by-Products and Value Addition—A Review. Resour. Conserv. Recycl. 2012, 66, 45–58.
- Cangussu, L.B.; Melo, J.C.; Franca, A.S.; Oliveira, L.S. Chemical Characterization of Coffee Husks, a By-Product of Coffea arabica Production. Foods 2021, 10, 3125.
- Iriondo-DeHond, A.; Aparicio García, N.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez Escobar, F.; Blanch, G.P.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204.
- Heeger, A.; Kosińska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage. Food Chem. 2017, 221, 969–975.
- Regazzoni, L.; Saligari, F.; Marinello, C.; Rossoni, G.; Aldini, G.; Carini, M.; Orioli, M. Coffee silver skin as a source of polyphenols: High resolution mass spectrometric profiling of components and antioxidant activity. J. Funct. Foods 2016, 20, 472–485.
- Bresciani, L.; Calani, L.; Bruni, R.; Brighenti, F.; Del Rio, D. Phenolic composition, caffeine content and antioxidant capacity of coffee silverskin. Food. Res. Int. 2014, 61, 196–201.
- Beltrán-Medina, E.A.; Guatemala-Morales, G.M.; Corona-González, R.I.; Padilla-Camberos, E.; Mondragón-Cortez, P.M.; Ruiz-Palomino, P.; Arriola-Guevara, E. Evaluation of the Analytical Conditions for the Determination of Chlorogenic Acid in Coffee Silverskin. Preprints.org 2019, 1, 2019090213.
- Martuscelli, M.; Esposito, L.; Di Mattia, C.D.; Ricci, A.; Mastrocola, D. Characterization of Coffee Silver Skin as Potential Food-Safe Ingredient. Foods 2021, 10, 1367.
- Husniati, H.; Oktiani, D. Chlorogenic Acid Isolation from Coffee as Affected by the Homogeneity of Cherry Maturity. Pelita Perkebunan 2019, 35, 119–124.
- Bravo, J.; Juániz, I.; Monente, C.; Caemmerer, B.; Kroh, L.W.; Paz De Peña, M.; Cid, C. Evaluation of Spent Coffee Obtained from the Most Common Coffeemakers as a Source of Hydrophilic Bioactive Compounds. J. Agri. Food Chem. 2012, 60, 12565–12573.
- Murthy, P.S.; Naidu, M.M. Protease production by Aspergillus oryzae in solid state fermentation utilizing coffee by-products. World Appl. Sci. J. 2010, 8, 199–205.
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Lehrbuch der Lebensmittelchemie, 6. Vollständig Überarbeitete Auflage; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 9783540732020.
- Perrone, D.; Donangelo, R.; Donangelo, C.M.; Farah, A. Modeling weight loss and chlorogenic acids content in coffee during roasting. J. Agri. Food Chem. 2010, 58, 12238–12243.
This entry is offline, you can click here to edit this entry!