Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Microbiology
Enterocin DD14 (EntDD14) is a two-peptide leaderless bacteriocin (LLB) produced by Enterococcus faecalis 14, a human strain isolated from meconium. Studies performed on EntDD14 enabled it to show its activity against Gram-positive bacteria such as Listeria monocytogenes, Clostridium perfringens, Enterococcus faecalis, and Staphylococcus aureus.
- Enterocin DD14 (EntDD14)
- leaderless bacteriocin (LLB)
- specific transport
- antibacterial and antiviral properties
- DdA
1. The Genetic and Synthesis Backgrounds of Enterocin DD14
The EntDD14 cluster encompasses 10 ORFs, namely ddABCDEFGHIJ [51]. Most of the genes present in the EntDD14 cluster are adjacent or overlap and in silico analysis suggested the presence of at least two operons. Thus, two putative transcriptional promoters and two putative terminators were detected. The first operon comprises ddA and ddB genes encoding the two-peptide LLB (DdA and DdB). Genes ddA and ddB are co-transcribed and their transcription is under the control of the putative P1 promoter, which is located upstream of the ddA gene (the P1 promoter presented a −35 (TTGAtA) and −10 (aATAAT) regions). The second contains the remaining genes ddCDEFGHIJ which encode two unknown proteins (DdC, D), membrane proteins YdbS and YdbT containing PHb2 domain DdE, F, and the ABC transporter (DdG, H, I and J) [51]. The transcription of this second operon seems to be dependent on the putative P2 promoter located upstream of the ddC gene (the P2 promoter presented a −35 (TTGttA) and a −10 (TATAtT) regions). The two putative ρ-independent transcriptional terminators designated T1 and T2 were identified between the 3’-end of ddB and the P2 promoter for T1 and the T2 at 163 nt downstream of the 3-end of the ddJ gene.
To gain insights into the transport of EntDD14 and immunity mechanisms of E. faecalis 14, different derivative strains were constructed and their phenotype has been examined. The data gathered enabled us to suggest the model depicted in Figure 1. Transport of EntDD14 outside the cell is essentially ensured by a channel formed by DdE and DdF proteins [53]. This channel is used as a primary non-ATP-dependent transport to externalize EntDD14, until a certain threshold of extracellular bacteriocin concentration (Figure 1A), the bacteriocinogenic strain switches to the ABC transporter composed of DdGHIJ, which is an ATP-dependent transport. This ABC system requires ATP to transport the bacteriocin in the opposite of the concentration gradient. These two transport systems, namely DdEF and DdGHIJ, and ABC transporters (DdGHIJ) should interact synergistically to control the flow of bacteriocin, through a mechanism that needs to be studied. When DdE and/or DdF proteins are not produced (Figure 1B), the bacteriocinogenic strain was unable to export EntDD14 outside of the cytoplasm, despite the expression of genes coding for the ABC transporters and those coding for active EntDD14 (ddAB). The intracellular accumulation of EntDD14 was to be toxic for the bacteriocinogenic strain as a loss of 1 log viability was registered in a mutant deficient in ddEF [52]. When the ABC transporter system (DdGHIJ) was altered by deleting one or more components (Figure 1B), the bacteriocinogenic strain was still able to produce EntDD14, but at a lower rate than the wild-type [51,53]. These data indicate the role of ABC transporter in the transport of EntDD14, but not as the essential transporter. Nonetheless, we have also established its involvement in the protection of the bacteriocinogenic strain against extracellular EntDD14 [53].
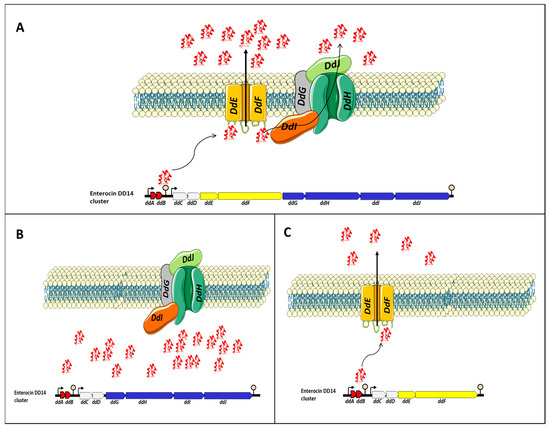
Figure 1. Proposed model for the transport of EntDD14. The legend is presented in the main text. (A) EntDD14 transport in the wild-type strain. (B) In the absence of DdE and/or DdF proteins, the strain was unable to export EntDD14 outside of the cytoplasm and an intracellular accumulation of EntDD14 was observed. (C) In the absence of one or more components the ABC transporter system (DdGHIJ), the bacteriocinogenic strain was still able to produce EntDD14, but at a lower rate than the wild-type.
2. Purification of Enterocin DD14
Despite their renewed interest in many applications, the purification of bacteriocins remains important in many cases, peculiarly in view of obtaining large amounts of purified and relatively clean active fraction which is generally difficult and costly [66,67]. The purification of bacteriocins, including leaderless ones, is usually carried out with a multistep procedure including ammonium sulfate precipitation, followed by one or more chromatography-based methods [66,67]. This strategy was largely utilized to purify bacteriocins devoid of leader peptide [38,68], such as enterocins L50A and L50B [20], A5-11A and A5-11B [37], and 62-6 [69]. Nonetheless, this strategy is time-consuming, expensive, and leads to low yields of purified peptides [67,70]. The ammonium sulfate precipitation enables a reduction in the volume of the culture supernatant and concomitantly increases the specific activity of the bacteriocin [71,72]. This step is usually not suitable for the purification of low molecular weight peptides due to the low precipitation yields and high saturation percentages [73,74,75], and this step requires a dialysis desalting phase, which can trigger significant loss of bacteriocins, because of their hydrophobic nature [75]. To overcome this drawback, easier methods of purification of bacteriocin were developed consisting of two-step-based methods. Such a simplified procedure was successfully applied for the purification of EntDD14 [33]. Accordingly, EntDD14 was purified using ion exchange chromatography and reverse phase HPLC, successively. To sum up, the producing strain, E. faecalis 14, was grown at 37 °C for 24 h with shaking (160 rpm), in an M17 medium supplemented with 5% glucose and buffered with 60 mM sodium phosphate (pH 6.3). After this period of incubation, the culture was centrifuged and the cell-free supernatant was incubated for 24 h at room temperature with CM Sephadex® C-25 resin, which was resuspended and equilibrated in distilled water. The resin was then washed with distilled water and 0.5 M NaCl. Thus, EntDD14 bound to the resin was eluted with 1.5 M NaCl. Removal of the salt from the solution containing EntDD14 was performed by running on PD MidiTrap G-10 columns. This procedure is routinely performed and usually leads to better purification yields. An additional drying step using a speedvac-type rotary concentrator could be applied with the objective to store this peptide at 4 °C until its use [33,76]. To characterize the molecular mass of EntDD14A and EntDD14B, an additional separative step by the HPLC method is required, due to their close masses which are, respectively, 5200.74 Da and 5206.41 Da [33]. These molecular masses are comparable to those reported for L50A and L50B [20], MR10A and MR10B [34], 7A and 7B [35] and A5-11A and A5-11B [37]. It should be, however, mentioned that the purification of bacteriocins could be achieved using other strategies such as membrane separation, which has emerged and applied for the purification of diverse bacteriocins [77,78,79], including some enterocins [80,81].
3. Antibacterial Spectrum of Enterocin DD14 and Potentiation of Antibiotic Activity
Leaderless bacteriocins, mainly enterocins, are active against a wide range of Gram-positive pathogenic bacteria including Listeria monocytogenes, Clostridium perfringens, Enterococcus faecalis and Staphylococcus aureus [18,20,33,34,82]. Related to its spectrum of activity, EntDD14 was proven to be active against MRSA strains in both in vitro [83] and in vivo [84]. The combination of antimicrobials is foreseen to be a valuable tool allowing for the alleviation of bacterial resistance [85]. Related to this, we first reported the capability of nisin to potentiate the activity of colistin against Gram-negative bacilli [86]. Next, we established the abilities of EntDD28 and EntDD93, two bacteriocins closely related to EntDD14, to augment the activity of erythromycin or kanamycin against the MRSA-S1 strain [82], and that of EntDD14 to potentiate the activity of methicillin against different MRSA strains [83]. Remarkably, MRSA-S1 was resistant to erythromycin and kanamycin; however, if any of these antibiotics was used in combination with EntDD14, EntDD28, or EntDD93, the MRSA-S1 target strain returned to the sensitive phenotype. In addition, an anti-biofilm activity of EntDD28 and EntDD93 was demonstrated on the human MRSA strains [82]. In another study, we established that EntDD14 loaded on alginate nanoparticles has a more potent activity across MRSA strains [83], or Clostridium perfringens [76]. It is of note that treatment of C. perfringens with such nanobiotics (bacteriocins loaded on alginate nanoparticles) has annihilated the expression of genes encoding some virulence factors [76]. Recently, a study carried out in our laboratory has established an anti-adhesive action of EntDD14 against several MRSA strains, noticeably across S. aureus USA300, which is known for its virulence traits and ability to colonize different surfaces [87]. In this study, we also unveiled the potential of EntDD14 to decrease the secretion of pro-inflammatory interleukins, IL-6 and IL-8, on human intestinal Caco-2 cells previously inflamed with bacterial lipopolysaccharide [87]. Generally active only against closely phylogenetically related microorganism, some LAB-bacteriocins, however, are exceptionally active against Gram-negative bacilli [88,89]. EntDD14 is devoid of such antibacterial activity. Nevertheless, when EntDD14 was used in combination with colistin and tested against a panel of Gram-negative bacilli such as Escherichia coli strains, we could observe a better spectrum of colistin, arguing for a potentialization of this antibiotic by EntDD14 [90].
4. The Antiviral Spectrum of Enterocin DD14
Bacteriocins have already been described for their antiviral activity [87,91], and these include EntCRL35 or GEn17 on Herpes Simplex Virus-1 (HSV-1) [92,93]. Moreover, a study based on a global simulation approach established that bacteriocins warrant more attention for their potential against SARS-CoV-2 viruses [94]. Experimental data reported that some bacteriocins such as plantaricin or nisin were able to interact with enveloped viruses and spike protein, respectively [95,96]. It has been observed that EntDD14 has an antiviral activity in vitro against enveloped viruses (personal communication).
5. Cytotoxicity of Enterocin DD14
Bacteriocins such as nisin are used in the food industry as a food preservative and have been extensively studied for their cytotoxic effects on different cell lines. For example, nisin has been shown to have an IC50 of less than 10 µg·mL−1 in intestinal Caco-2 cells after 24 h of contact, and an IC50 of around 150 µg·mL−1 in Vero SF cells (monkey epithelial cells from kidney) [97]. In comparison, enterocin AS-48 (produced by Enterococcus faecalis UGRA 10) showed no cytotoxicity up to 200 µg·mL−1 on melanoma cell line A2058 [98]. In this context, EntDD14 was studied for its cytotoxic effects on several cell lines. First, the cytotoxicity of the purified enterocin DD14 was assessed using the IPEC-1 porcine intestinal epithelial cell line. After 24 h of contact, a dose-dependent decrease in IPEC-1 cell viability was observed when exposed to EntDD14. At concentrations of 50 μg·mL−1 and 100 μg·mL−1, reductions of 9.6% and 20% in cell viability were noted, respectively [32]. The cytotoxic effect of EntDD14 on two cell lines of human origin, intestinal Caco-2 cells, and HT29 mucus-producing cells was studied [76]. Thus, no apparent cytotoxicity was observed when EntDD14 was in contact with HT29 cells for 4 h and 24 h incubation and the two concentrations were tested (60 and 120 µg·mL−1). Regarding Caco-2 cells, the viability remained approximately 95% when exposed to a concentration of 120 µg·mL−1 of EntDD14 for 24 h [76]. Finally, another study (data under publication elsewhere) has shown the cytotoxic effects of EntDD14 on two cell lines, VERO E6 cells (derived from the kidney of an African green monkey) and Huh 7 cells (human hepatocellular carcinoma). For both cell lines, the viability decreased to 75% after 24 h incubation with EntDD14 at 120 µg·mL−1 (Submitted). To conclude, EntDD14 was found to be less cytotoxic than nisin.
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics12071188
This entry is offline, you can click here to edit this entry!
