Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
Skin pigmentation ensures efficient photoprotection and relies on the pigment melanin, which is produced by epidermal melanocytes and transferred to surrounding keratinocytes. While the molecular mechanisms of melanin synthesis and transport in melanocytes are now well characterized, much less is known about melanin transfer and processing within keratinocytes.
- melanin
- melanocyte
- keratinocyte
- melanosome
- melanocore
1. Introduction
The skin is the largest organ of the body and plays fundamental roles in water balance, regulation of body temperature, and protection against foreign agents and ultraviolet radiation (UVr) [1]. The upper layer of the skin — the epidermis — is mainly comprised of keratinocytes, which account for >80% of the cells in this layer. Keratinocytes stratify into five different strata according to their differentiation status: stratum basale, stratum spinosum, stratum granulosum, stratum lucidum (only visible in thick skin), and stratum corneum [2,3]. The epidermis also contains melanocytes, which are specialized cells derived from the neural crest during embryonic development [4]. Melanocytes are dispersed throughout the stratum basale and can extend dendrites to contact up to 40 keratinocytes, forming “epidermal-melanin units” (Figure 1A) [5,6]. The function of melanocytes is to synthesize the pigment melanin, subsequently transferring it to keratinocytes [7]. There are two types of melanin: black/brown eumelanin and yellow/red pheomelanin [8,9]. These are synthesized in melanocytes within melanosomes, which are lysosome-related organelles (LROs), as they are endosomal-derived, contain lysosomal markers, and are acidic in the early stages of their biogenesis [10]. Melanosomes display four distinct stages of maturation: In stages I and II, they are non-pigmented, initially resembling multivesicular endosomes. While pheomelanosomes maintain a spherical shape, eumelanosomes acquire an elliptic shape due to internal fibril nucleation of the premelanosomal protein (PMEL) [10]. Melanogenic enzymes required for eumelanin synthesis, including tyrosinase and tyrosinase-related proteins (TYRP1/2), are recruited to stage III melanosomes, and melanin starts to progressively deposit onto PMEL fibrils until melanosomes achieve full pigmentation (stage IV) [10,11]. Mature melanosomes accumulate at melanocyte dendrites and are then transferred to keratinocytes. After being internalized by keratinocytes, melanin is processed, i.e., trafficked, before accumulating in the supranuclear area, where it exerts its function of protecting the DNA of these cells from UVr [12]. Thus, skin pigmentation results from several processes that involve melanin synthesis in melanocytes, transfer from melanocytes to keratinocytes, and processing within keratinocytes. The molecular machinery involved in melanin synthesis and transport within melanocytes is now well characterized, but the mechanisms of melanin transfer remain controversial and poorly characterized. Moreover, only a few studies have addressed how melanin processing occurs within keratinocytes, and much remains to be uncovered.
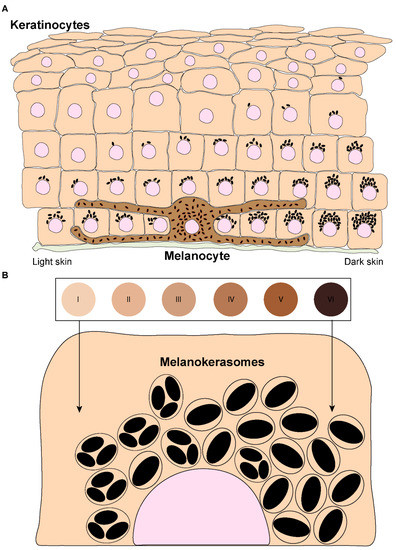
Figure 1. Melanin distribution throughout the epidermis and organization within keratinocytes from distinct phototypes. (A) Melanin dispersion in the epidermis is achieved through the formation of epidermal-melanin units. The accumulation of melanin is higher in the basal layers, regardless of the skin phototype (represented by the numbered circles and labeled from I — lower/lighter phototype — to VI — higher/darker phototype). However, in lower phototypes (lighter skins), less melanin is concentrated in the basal layer, whereas in higher phototypes (darker skins), higher levels can be found in the basal layer, and melanin is also present in the immediate suprabasal layers. (B) Melanin organizes differently within keratinocytes derived from different phototypes. In lower phototypes, melanin is mainly stored in clusters of several melanin granules within a single membrane organelle, while higher phototypes prominently accumulate single granules in single membrane organelles.
2. Melanin Transfer Models
Melanin must be transferred to keratinocytes to exert its photoprotective function in these cells. The process by which melanin transfer occurs has been a controversial topic since as far back as the 1960s, when melanin was first observed being transferred from a melanocyte to an epithelial cell (keratinocyte) [13]. As many as four models have been proposed to explain how melanin is transferred from melanocytes to keratinocytes, even though some could be variations of the same: (I) Cytophagocytosis of melanocyte dendrite tips, containing melanosomes, by keratinocytes; (II) Membrane fusion between keratinocytes and melanocytes and direct transfer of melanosomes; (III) Shedding of melanosome-laden globules by melanocytes and subsequent phagocytosis by keratinocytes; and (IV) Exocytosis of the melanin core by melanocytes, followed by phagocytosis by keratinocytes (Figure 2). These are subsequently summarized:
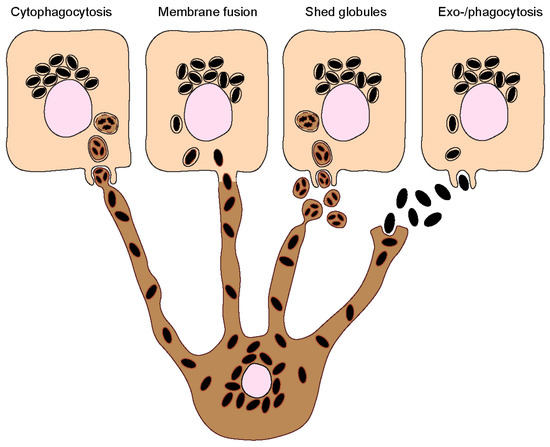
Figure 2. Melanin transfer from melanocytes to keratinocytes. Four different models have been proposed to explain the mechanism(s) of melanin transfer from melanocytes (dark-colored) to keratinocytes (light-colored), including the cytophagocytosis of melanocyte dendrites by keratinocytes; the direct fusion of melanocyte and keratinocyte membranes; the shedding of melanosome-laden globules by melanocytes; and the coupled exocytosis/phagocytosis of the melanin core. Despite the evidence published using different models, recent studies using human and mouse cell lines, as well as more complex models such as reconstructed human skins/epidermises and skin biopsies, have supported the transfer of melanin in globules or as melanocores.
-
Cytophagocytosis of melanocyte dendrites by keratinocytes.
This transfer mode was first proposed after observations made by electron microscopy (EM) of melanocyte dendrite tips within keratinocytes in vitro [14]. The process can be divided into four steps: Firstly, a melanocyte dendrite tip comes into contact with the keratinocyte plasma membrane and is subsequently engulfed by it; secondly, the melanocyte dendrite tip is pinched off, leading to the formation of a cytoplasmic vesicle filled with melanosomes; and thirdly, the compartment where melanin resides, which is theoretically limited by three membranes of different origins — the innermost one corresponding to the membrane of the melanosome and the plasma membranes of the melanocyte and the keratinocyte — fuses with the lysosome to form a phagolysosome. This leads to the degradation of the luminal membranes [15]. Lastly, the phagolysosome fragments into smaller vesicles containing aggregates or single melanin granules dispersed in the cytoplasm [16].
- 2.
-
Fusion of melanocyte and keratinocyte membranes.
This model postulates that the plasma membranes of the melanocyte and keratinocyte fuse, allowing the formation of a conduit (e.g., filopodia or nanotubes) between the cytoplasm of the neighboring cells, through which melanosomes are transported [17]. As in the cytophagocytosis model, keratinocytes receive membrane-bound melanosomes. However, melanin within keratinocytes was shown to be devoid of melanosome markers [18,19]. A possible explanation for this discrepancy is that the melanosome membrane is degraded inside keratinocytes by the endolysosomal system. It should be noted that this model and the cytophagocytosis one could represent variations of the same. Indeed, a filopodia-cytophagocytosis model was proposed, in which melanocytes extend filopodia that bind to the keratinocyte plasma membrane and are then pulled and subsequently phagocytosed by the keratinocyte [20,21].
- 3.
-
Shedding of melanosome-laden globules.
This model proposes that the blebbing of the melanocyte membrane leads to the formation and release of globules (1–3 µm in size) filled with melanosomes, which are then phagocytosed by keratinocytes [22,23]. Recently, other studies showed that globules filled with melanosomes are secreted from primary human melanocyte dendrites to the cell culture medium [24]. These melanosome-laden globules, when isolated from the medium and incubated with primary human keratinocytes, are internalized and processed in a similar manner as postulated by the cytophagocytosis model [25]. Indeed, according to this model, upon internalization, melanin is surrounded by three membranes. After processing, single membrane vesicles containing melanin are dispersed inside keratinocytes [25].
- 4.
-
Coupled exocytosis/phagocytosis of the melanin core.
This transfer model predicts that melanosomes fuse with the melanocyte plasma membrane, leading to the exocytosis of the naked melanin core, termed melanocore, to the extracellular space and its subsequent internalization by keratinocytes. Consequently, melanocores become surrounded by a single membrane derived from the keratinocyte plasma membrane upon internalization. The first evidence supporting this model came with the observation of extracellular melanin in human hair and skin, which was hypothesized to be internalized by keratinocytes in aggregates or individual granules [26].
The different models are supported by evidence obtained in various experimental systems, namely primary cells, cell lines, and tissue explants from different species, including mice, guinea pigs, chickens, frogs, and humans. In co-cultures of melanocytes and keratinocytes isolated from black guinea pig ears, evidence for the cytophagocytosis transfer model was reported [14]. In frog skin cells and tissues, melanin transfer was described as occurring through shed melanin-laden globules or exo-/phagocytosis of melanosomes, as extracellular melanin was found to be membrane-bound [23]. In chicken embryonic skin, melanin was proposed to be transferred via shed globules [28]. In mice, melanin transfer occurs in the hair follicles, and evidence for the models of exo-/phagocytosis and shed globules has been described [29]. Regarding the different systems used, most studies were performed with cultured melanocytes, either alone or in co-culture with keratinocytes. However, in the skin epidermis, melanin transfer occurs between melanocytes and keratinocytes arranged in epidermal-melanin units. Therefore, models that reproduce this arrangement should be favored. Additionally, the use of melanoma cell lines for the study of the molecular mechanisms of pigment transfer should be performed with caution, as they can show alterations in melanin production, processing, and secretion [30]. Importantly, the model of exo-/phagocytosis is the one that collected more supporting evidence from the analysis of human skin sections and reconstructed human skin/epidermis in vitro models [19,31]. The shed globules is the other model that has been more explored. Furthermore, the four models can be essentially divided into the one that postulates the transfer of naked melanin (melanocore) and the ones that predict the transfer of membrane-bound melanosomes. It should be noted that it cannot be excluded that several mechanisms of melanin transfer co-exist, even in the same organism, as adaptations to different physiological or pathological conditions. To solve these long-lasting questions, a better understanding at the cellular and molecular levels of the pathways and regulators of melanin exocytosis from melanocytes and internalization by keratinocytes is essential.
This entry is adapted from the peer-reviewed paper 10.3390/ijms241411289
This entry is offline, you can click here to edit this entry!
