Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Acetone is a well-known volatile organic compound that is widely used in different industrial and domestic areas. However, it can have dangerous effects on human life and health. Thus, the realization of sensitive and selective sensors for recognition of acetone is highly important. Among different gas sensors, resistive gas sensors based on nanostructured metal oxide with high surface area, have been widely reported for successful detection of acetone gas, owing to their high sensitivity, fast dynamics, high stability, and low price.
- acetone
- gas sensors
- metal oxide-based sensor
- sensitivity
- selectivity
- sensing mechanism
1. Introduction
As a general rule, chemical compounds containing at least one carbon (C) and one hydrogen (H) atoms in their molecular structure are called organic compounds [1]. They are referred as volatile organic compounds (VOCs) when they turn volatile at ambient temperature [2]. As an important member of VOCs, acetone (CH3COCH3; IUPAC name is propanone) is a widely used substance [3].
Acetone (see the molecular structure in Figure 1) has a molecular weight of 58.08 g/mole, density of 0.79 g/cm3 at 20 °C, and an intense odor, and it is an extensively used solvent in industry and is also found in many common domestic commodities. However, it can be easily inhaled, resulting in serious effects on human health [4]. Acetone concentrations higher than 173 ppm can severely affect the central nervous system and damage important organs of the body [5]. Furthermore, damage to eyes and nose are other effects of long-term exposure to acetone [6]. Accordingly, the occupational threshold limit value for acetone has been set to 250 ppm, considering an 8 h time weighted average [7]. Along with the negative effects to human body, it is a flammable substance with low explosive limit (LEL) of 2.6% and upper explosive limit (UEL) of 12.8% [8].
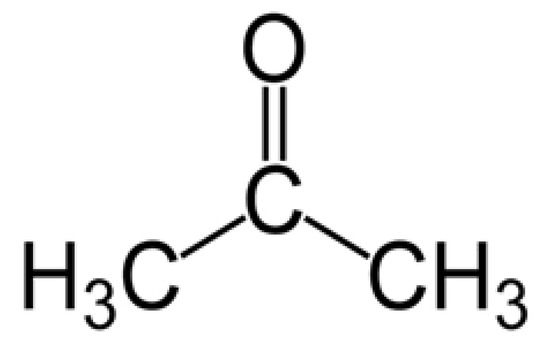
Figure 1. Chemical structure of acetone.
VOCs are generated either from inside the body (endogenous VOCs) or from external sources such as food ingestion and environmental exposure (exogenous VOCs). The exhaled human breath comprises ~3500 different VOCs [9] and the analysis of VOCs in breath gas may become a promising non-invasive tool and simple health check method that can be conducted both at home and in a medical facility for medical diagnosis and for monitoring the success of therapy [10][11]. For example, breath analysis can be used for the early diagnosis of diseases, such as lung cancer [12], congestive heart failure [13], diabetes [14], and asthma [15]. In addition, high concentration of hydrogen gas in the breath shows a trace of small intestinal bacterial overgrowth (SIBO) in patients suffering from symptoms, such as nausea, bloating, vomiting, diarrhea, malnutrition, weight loss, and malabsorption [16]. Moreover, as ammonia concentration drops after dialysis, a highly sensitive ammonia sensor can be used for a real-time and low-cost breath ammonia sensor for the daily tracking of hemodialysis patients [17]. Acetone is considered an important type-1 diabetes biomarker, and it is reported that the exhaled breath of diabetic people contains a higher concentration value of acetone (>1.8 ppm) compared to healthy people (0.3–0.9 ppm) [18]. Therefore, monitoring breath acetone can be considered as a useful way to follow patients on a prescribed diet regime, as well as to monitor diabetic patients [19]. Furthermore, there is a correlation between acetone and blood glucose level, and thus, its monitoring can be used for insulin management [20].
Concentration of VOCs can be measured using standardized methods such as gas chromatography mass spectrometry (GC-MS) [21], high-performance liquid chromatography [22], and proton transfer reaction mass spectrometry (PTR-MS) [23]. These techniques have high sensitivity and precision for detection of various VOCs. Nevertheless, they are bulky, complex, expensive, time consuming, and need skilled operators for monitoring gases [24]. Therefore, there is a need for small, portable, and fast dynamic devices that can easily detect acetone vapor [25][26].
Gas sensors are electrical devices that can produce an electrical signal in the presence of target gases [27]. A practical gas sensor must be highly sensitive, selective, stable, and fast, with low prices, and requires low operational power [28]. Furthermore, gas sensors should have a high signal-to-noise ratio (S/N), which indicates the relative gas signal intensity over noise intensity. The electrical noise of gas sensors can be determined by measuring the average resistance fluctuation before introduction of target gas. Generally, a low noise level can be induced by a high electrical conductivity [29]. It is accepted that an S/N value of three is needed for estimating the limit of detection (LOD) [30]. LOD is defined as three N/S, where N stands for the root mean square noise and S refers to the slope of the calibration curve of gas sensor [31].
So far, different gas sensors such as field-effect transistors (FETs) [32], optical [33], electrochemical [34], surface acoustic wave [35], cataluminescence [36], microwave-based [37], quartz crystal microbalance [3], microcantilever [38] mixed potential [39][40], photonic waveguide [41], and resistive-based [42][43] have been introduced in the literature.
2. Metal Oxide-Based Gas Sensors: Introduction, Design, and General Mechanism
Metal oxide-based gas sensors are often fabricated by coating a sensing layer over an insulating substrate and then providing it with interdigitated electrodes [44]. A heater is also included in many sensors in order to raise the temperature of the gas sensor up to 500 °C [45]. Micromachined gas sensors can be used for attaining high temperatures [46]. Furthermore, they are fabricated from nanoscale materials, which have a high surface area and high reactivity to target gases [47][48]. In addition, it is well known that morphology is one of the most important factors affecting the gas sensing performance. Therefore, different morphologies, such as nanoparticles [49], nanorods [39], hollow spheres [50], nanowires (NWs) [51], hierarchical [52], porous spheres [53], flowers [54], and dumbbell-like [55] morphologies have been reported for acetone or other gas sensing studies.
The general mechanism of sensing for metal oxide-based acetone gas sensors is as follows. Initially, in air, due to high electron affinity of oxygen molecules (0.43 eV [56]), they get adsorbed on the surface of the sensor and take electrons from the sensing layer. Accordingly, depending on the operating temperature, different oxygen ion species will be available on the surface of gas sensors as demonstrated by the following equations [57]:
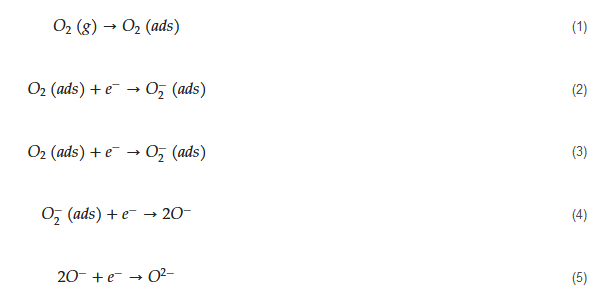
The tested gas species depend on the sensing material and sensing temperature. However, generally, molecular ions are stable below 150 °C, while other forms are stable at higher temperatures [58][59]. Due to abstraction of electrons and depending on the n-type or p-type nature of gas sensor, an electron depletion layer or a hole accumulation layer, respectively, will be generated on the surface of gas sensor. Since the majority of charge carriers in n-type sensors are electrons and those in p-type gas sensors are holes, the resistance of n-type sensors increases relative to vacuum, while the resistance of p-type gas sensors decreases relative to vacuum. When acetone gas is injected into a gas test chamber, it will react with oxygen ions that were already adsorbed and electrons will be released as follows [60][61]:
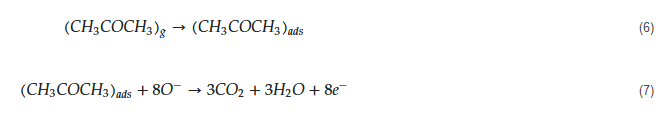
The released electrons are injected in to the sensing layer and the width of electron depletion layer of an n-type sensor or hole accumulation layer of a p-type sensor is decreased. Accordingly, the resistance of n-type sensors decreases and that of p-type gas sensors increases upon exposure to acetone. The response of gas sensor to acetone gas, which is a reducing gas, is usually defined as Ra/Rg for n-type gas sensors and Rg/Ra for p-type gas sensors, where Ra is the resistance of gas sensor in the presence of air and Rg is the resistance of gas sensor in the presence of acetone gas.
Figure 2 shows the general acetone sensing mechanism for pristine n-type and p-type metal oxides. For noble metal-decorated or heterojunction metal oxide-based gas sensors, in addition to above mechanism, formation of Schottky contacts, formation of n–n, n–p, or p–p heterojunctions, and catalytic effects of noble metals are other mechanisms which can further enhance the response of gas sensor and will be explained in the subsequent sections of this entry.
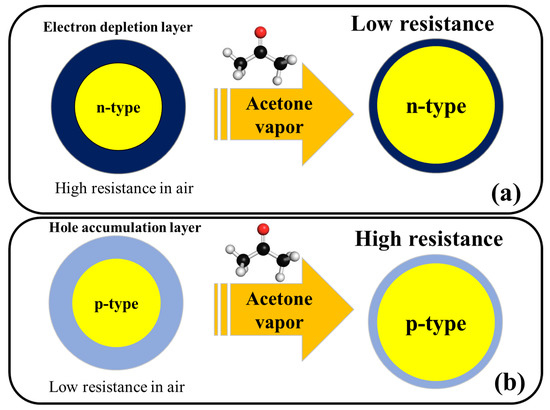
Figure 2. Schematic illustration of acetone sensing mechanism in (a) n-type metal oxides and (b) p-type metal oxides.
In general, nanostructured materials such as 0-dimensional, 1-dimensional, and 2-dimensional metal oxides have higher sensitivity relative to their bulk counterparts. In fact, such materials not only have a high surface area which greatly enhances the response of gas sensors due to offering of more adsorption sites for the target gas molecules but they also have many contact points which act as potential sources for the resistance modulation. Another advantage of nanostructured metal oxides stems is from the fact that when the size of materials reaches to the Debye length (λD), adsorption of target gas molecules on the surface of sensor leads to great modulation of electrical resistance, leading to the appearance of a high sensing signal [62].
3. Pristine Acetone Gas Sensors
In 1991, bismuth ferrite (BiFeO3) nanoparticles (NPs) were studied for sensing applications and it was reported that sensing performance was related to the chemical composition [63]. Moreover, recently, BiFeO3 NPs were used for acetone sensing and they showed a response (Rg/Ra) of 10–12 ppm acetone gas at 350 °C with a fast response time of 25 s and a recovery time of 17 s [64]. In another study, p-type praseodymium ferrite (PrFeO3) was used for sensing studies. Electrospun hollow PrFeO3 nanofibers with a high surface area of 33.74 m2/g showed a high response (Rg/Ra) of 141–200 ppm acetone at 180 °C [65].
Semiconducting spinel ferrites (AFe2O4), in which the transition metal cation “A” was included into the lattice of the parent structure of (Fe2+Fe23+O4) [66][67][68][69], had environmental friendliness, low cost, and excellent stability [70][71][72]. Therefore, they were studied for acetone sensing applications [73][74]. For example, super fine porous NiFe2O4 spheres were fabricated using a solvothermal method. The surface area of the product was 20.04 m2/g with an average pore size of ~8.9 nm. The fabricated sensor revealed a high response (Rg/Ra) of 27.4–100 ppm acetone with a fast response time of 2 s at 250 °C. The sensor showed a moderate selectivity to acetone. In addition, the response to acetone was significantly decreased with an increase of relative humidity, which limits its real-world applications [18].
4. Doped Acetone Gas Sensors
4.1. Binary Metal Oxide Gas Sensors
Doping is a widely used strategy to enhance acetone sensing properties [75][76]. In an interesting study, the bare and W-doped NiO hierarchical hollow spheres were synthesized by a hydrothermal method. The sensor with 4.0 at% of W-doping showed a response (Rg/Ra) of 198.1–100 ppm acetone at 250 °C, while the response of bare sensor was 139 times lower. Higher response of doped sensor was related to the lower crystalline size as a result of W-doping, higher surface area (217.2 m2 g−1), and higher concentrations of hole carriers due to W-doping, which created additional holes in the NiO [77].
2D Sn-doped ZnO ultrathin nanosheet networks were synthesized by a hydrothermal method [78]. At 320 °C, which was reported as the optimal sensing temperature, the sensor showed a response (Ra/Rg) of 5.6–50 ppm acetone gas. Role of Sn-doping was attributed to the creation of more defect in crystal by substitution in ZnO crystal structure.
La2O3 is rarely used for acetone sensing studies. Xu et al. [79] synthesized bead-like 1 wt% La2O3-doped ZnO NFs through electrospinning for acetone sensing studies. The gas sensor showed a response (Ra/Rg) of 48–100 ppm acetone gas at 340 °C. The excellent gas sensing properties caused by La doping were related to the larger surface area of gas sensor, the increased adsorption capacity of O due to presence of La2O3, and the useful hetero contacts between n-type ZnO and p-type La2O3.
Co-doped ZnO NFs were produced through an electrospinning process followed by an annealing process [80]. At optimal sensing temperature of 360 °C, the response (Ra/Rg) of 0.5 wt% Co-doped ZnO NFs to 100 ppm acetone was ~16, while that of pristine sensor was only 4.4. Additionally, response and recovery times of optimal sensor were 4 and 6 s, respectively. High response to acetone was due to the high surface area of synthesized NFs and presence of plenty of NF–NF junctions in the netlike structure. Furthermore, Co-doped sensor showed a decreased NF diameter relative to pristine ZnO NFs. Finally, catalytic effect of Co3O4 led to easy oxidation of acetone, contributing to the sensor signal.
Hollow NFs can provide significantly higher surface area relative to NFs and therefore, it can be expected to have high response to acetone gas. Y-doped SnO2 hollow NFs were synthesized through electrospinning technique. The 0.4 wt% Y-doped SnO2 hollow NFs showed a response (Ra/Rg) of 174–500 ppm acetone gas at 300 °C [81]. High sensing performance to acetone gas was related to the high surface area of gas sensor (29.46 m2/g), presence of many small nanograin–nanograin junctions on the surface of SnO2 hollow NFs, and catalytic effect of surface “Y” clusters, which enhanced gas response to acetone gas.
Like other rare earth elements, Eu rarely has been studied for gas sensing studies. In a study by Jiang et al. [82], Eu-doped SnO2 NFs were produced by electrospinning for acetone sensing studies. The sensor was able to detect even 0.3 ppm acetone, making it a potential candidate for the breath diagnosis of diabetes. When Eu3+ ions diffused into the SnO2 lattice and substituted Sn4+ ions, the mismatch in ionic radius between Eu3+ (0.947 Å) and Sn4+ (0.69 Å) resulted in the lattice distortion and defects, leading to enhanced gas response. Furthermore, Eu2O3 was able to accelerate the reactions between acetone molecules and absorbed oxygen species to release electrons to the conduction band, leading to an improved gas response.
4.2. Ternary Metal Oxide Gas Sensors
P-type LaMnO3+δ (LMO) perovskites oxides have fascinating properties owing to excess oxygen as well as coexistence of Mn3+/Mn4+ ions with different oxidation states [83]. In order to enhance gas sensing properties of the LMO gas sensor, yttrium-doped LMO NPs (namely, La0.85Y0.25MnO3+δ NPs) were synthesized via a sol–gel method. It showed a response (Rg/Ra) of ~26–500 ppm acetone gas at 300 °C. However, its response to ethanol was almost 5 times lower than that of the pristine gas sensor. Due to doping of Y3+ and in order to maintain the charge neutrality, the Mn4+/Mn3+ ratio was increased, and accordingly, more oxygen species were adsorbed on the surface of gas sensor. Due to the p-type nature of gas sensor, initial resistance of the sensing layer was significantly decreased, and upon exposure of sensor to acetone vapor, the resistance significantly increased, leading to high response of sensing layer to acetone [84].
P-type ytterbium ferrites, with a general formula of YbFeO3, have high stability, and their properties can be tuned through metal doping. In an interesting study related to YbFeO3, 20 at% Ca-doped YbFeO3 (Yb0.8Ca0.2FeO3) was synthesized via a sol–gel process for sensing study. Interestingly, the response to acetone gas was increased in humid air at room temperature. The presence of –OH groups on the surface of the sensor was reported to be the reason for such an increase. However, it seems that more studies are needed to find such a strange behavior of this gas sensor [85].
5. Decorated/Loaded Acetone Gas Sensors
SnO2, is a metal oxide n-type semiconductor (Eg = 3.6 eV) with high stability, low synthesis costs, and high sensing properties due to its high mobility of electrons (160 cm2/V·s) [86][87]. SnO2 hierarchical structures loaded with Sm2O3 (0.5, 1, 2.5, and 4 mol%) were synthesized by a hydrothermal method and subsequent isometric impregnation technique. It was revealed that the 2.5 mol% Sm2O3/SnO2 gas sensor showed a response (Ra/Rg) of 41.14–100 ppm acetone gas at 200 °C, with a limit of detection of 100 ppb. Upon replacement of Sm3+ in SnO2 lattice, oxygen vacancies were formed and much higher target gases were adsorbed on the surface of the gas sensor. Furthermore, the catalytic effect of Sm2O3 was reported to be important for acetone sensing [88].
TiO2 NPs were decorated on In2O3 NWs for acetone sensing studies [89]. At 250 °C, the fabricated sensor showed a response (Ra/Rg) of ~34–10 ppm acetone which was higher than that of ethanol and other interfering gases. Enhanced sensing response to acetone was due to the changes in the surface depletion layer width and potential energy barrier width of In2O3 NWs.
Rh2O3-decorated WO3 NFs were synthesized via an electrospinning process for acetone sensing studies [90]. The sensor showed a response (Ra/Rg) of 41.2–5 ppm acetone gas at 300 °C. When Rh2O3-decorated WO3 NFs were exposed to target gas, due to reduction of Rh2O3 NPs, the width of depletion region inside of WO3 was decreased, leading to enhanced gas response towards acetone. Furthermore, presence of oxygen vacancies contributed to generation of a high sensing signal.
Not only metal oxides but also noble metals can be decorated on the surface of sensing layers. Different noble metals have been decorated on the sensing layer for acetone sensing studies [91][92]. Hollow porous Fe2O3 nanocubes were synthesized, and subsequently, Pt NPs were loaded on the surface of Fe2O3 nanocubes by means of a reduction process. The sensor had a response (Ra/Rg) of 25.7–100 ppm of acetone at 139 °C. Both chemical sensitizations and electronic sensitization of Pt had a high contribution to the sensing signal. Oxygen molecules were dissociated on the surface of Pt and subsequently spilt over the surface of Fe2O3, leading to high adsorption of oxygen ions. The surface of Pt NPs preferentially adsorbs oxygen molecules in air and then spill the oxygen species over to the Fe2O3, owing to the catalytic promotion of Pt. Furthermore, the electrons were transferred from Fe2O3 to Pt, and Schottky barriers were formed in air. In an acetone atmosphere, the height of the Schottky barrier significantly decreased, leading to an enhanced response to acetone. Furthermore, it was reported that in air, some PtOx can be formed and the resultant p–n heterojunctions can enhance resistance modulation in the presence of air [93].
6. Composite Acetone Gas Sensors
In the literature, there are many studies related to acetone sensing properties of composite materials. This is due to the fact that in composite materials, plenty of heterojunctions can form, leading to significant modulation of electrical resistance [54]. However, here, the researchers just report the results of the most interesting acetone gas sensors in terms of design or gas sensing performance.
Electrospun Pt@In2O3 core–shell composite NWs were prepared for acetone sensing studies [94]. The sensor showed a highly improved response, short response and recovery time of 14 and 16 s, respectively, for 1 ppm of acetone, and high selectivity and stability compared with a sensor based on pristine In2O3 NWs due to the increase in surface resistance and the presence of heterojunctions. In addition, detection limit was low as 10 ppb, which was much lower than the concentration level of 1.8 ppm in the exhaled breath of diabetic patients. The influence of the large amount of moisture was greatly weakened by using the molecular sieve as a moisture filter layer, leading to much improved sensitivity to acetone in clinical sample detection.
WOx≤3 oxide has different nonstoichiometric phases, namely, WO2, WO2.72, WO2.8, and WO2.9. In general, these phases have a lot of oxygen vacancies, which promote the adsorption of target gases on the surface of gas WOx≤3-based gas sensors [95]. In addition, MXenes are a new class of 2D transition metal of carbides/nitrides, that are rich with functional groups such as –O, –OH, and –F, which are beneficial for gas sensing studies [96]. In this regards, a series of WO2.72(W18O49)/Ti3C2Tx composites were solvothermally prepared for sensing studies towards acetone [97]. The W18O49 nanorods were distributed on the surface of Ti3C2Tx nanosheets, leading to an increase of adsorption sites relative to pristine sensors. The W18O49/Ti3C2Tx–2% sensor showed a response (Ra/Rg) of 4.2–5 ppm acetone gas at 300 °C, which was higher than other gas sensors with different amounts of Ti3C2Tx as well as pristine gas sensors. First, due to presence of functional groups on the surface of Ti3C2Tx, the acetone molecules were effectively adsorbed on the surface of the gas sensor. Second, due to metallic nature of for the Ti3C2Tx, heterojunctions were creased on the interfaces between Ti3C2Tx and W18O49, and in the acetone atmosphere, the modulation of heterojunction potential barrier led to enhanced sensing response. Third, for the compositions with a higher amount of Ti3C2Tx, some –F groups were attached to the surface, leading to decrease of gas response. Finally, too much Ti3C2Tx caused the stacking of the nanosheets, and this decreased the adsorption sites of gas sensor.
CuO with p-type sensing properties has been used for sensing studies due to its low synthesis cost and catalytic effects [98]. A very low power consumption gas sensor was prepared using Fe2O3/CuO-based nanostructures which were fabricated by direct ink writing (DIW) on top of the surface of an insulating (glass) substrate. Due to the presence of copper oxides and iron oxides, many heterojunctions were produced on the surface of glass substrate. The power consumption of gas sensor at 300 °C was only 0.26 μW, and the gas sensor showed a response of 50% to 100 ppm acetone vapor. Formation of Fe2O3/CuO heterojunctions in air and subsequent modulation of potential barriers in acetone vapor were the main reasons for acetone sensing mechanism [99].
In comparison to the dense and thick films, three-dimensional (3D) ordered composites have a large surface area, which guarantees the high number of adsorption sites, leading to higher sensing properties. Moreover, because of having a porous structure and open channels, surrounding gases can diffuse into the deep part of gas sensor, contributing to high gas response. In this context, the 3D inverse opal (3DIO) composite of ZnO-Fe3O4 was prepared using a template method [100]. The ratio of Fe/Zn atoms was varied to find the optimal gas sensor. For the gas sensor with a Fe:Zn atom ratio of 2:10 at 485 °C, a response (Ra/Rg) of 47–50 ppm acetone vapor was reported. It was also reported that the amount and size of mesoporous increased with the increase of Fe. As a result, the surface area increased and more gas molecules were able to be adsorbed on the surface of sensor. However, for the sensor with Fe:Zn, atomic ratio of 3:10, the structure was damaged and sensing properties decreased. Furthermore, ZnO/Fe2O3 heterojunctions significantly enhanced the sensing properties. ZnO/Fe2O3 heterojunctions were initially formed in air upon intimate contact between ZnO and Fe2O3. Subsequently, by exposure of the gas sensor to acetone vapor, the height of heterojunction barriers was decreased, leading to modulation of sensor resistance. This ultimately contributed to the gas response.
The two-dimensional (2D) heterostructure of the C3N4-SnO2 nanocomposite sensors with a high surface area of 57.13 m2/g were prepared for gas sensing studies [101]. The sensor showed a response (Vg/Va) of 29–100 ppm acetone gas at a sensing temperature of 380 °C with fast response/recovery times (7 and 8 s, respectively). In addition, it was possible to detect as low as 67 ppb acetone, which is way below the exhaled breath concentration of diabetic people. Excellent acetone sensing properties were related to the exchange of electrons from SnO2 to C3N4 and formation of heterojunctions. Furthermore, the large surface area of C3N4 layer provided high number of adsorption sites for target gases. In addition, good selectivity of gas sensor was related to the large dipole moment of acetone arising from the C–C=O group of acetone. Dipole moment of acetone was higher than that of ethanol (1.69 D), methanol (1.70 D), NO (0.159 D), NO2 (0.316 D), NH3 (1.471 D), and CO (0.112 D) gases [102], leading to more interaction between acetone and the sensing layer.
NiO/ZnO composites were prepared by decoration of NiO NPs on the surfaces of ZnO hollow spheres using a solvothermal technique. The response (Ra/Rg) of NiO/ZnO composite sensor to 100 ppm acetone was ~29 at 275 °C, and both response and recovery times were 1 and 20 s, respectively. In dry air, the p–n junction between n-type ZnO and p-type NiO was produced. As a result, the resistance of the sensor was increased in air. In the acetone atmosphere, due to release of electrons to the surface of gas sensor, the resistance significantly decreased and a response appeared.
Defects and functional groups presenting on the graphene oxide (GO) surface act as high-energy adsorption sites for the gas molecules and can increase the response to acetone gas. In this regards, ZnO nanosheet/GO nanocomposites were prepared for detection of acetone [103]. The sensor with 10 wt% GO showed the highest sensing properties and a response (Ra/Rg) of 35.8–100 ppm acetone was observed at 240 °C. Presence of p-GO and n-ZnO potential barriers, high surface area of 2D nanocomposite, and presence of functional groups on the surface of GO were the main factors contributing to the sensor signal.
Organic–inorganic hybrid materials generally have enhanced sensing properties such as operating temperature and response time relative to pure materials due to a synergic effects between the different materials [104]. A polyaniline (PANI)/SnO2 hybrid material was prepared by a hydrothermal method for acetone sensing studies. The sensor revealed the highest response (Rg/Ra) of 1.68–800 ppm acetone gas at 60 °C. PANI is a p-type semiconductor and SnO2 an n-type, so that in intimate contacts, formation of p–n heterojunctions can create potential barriers in air. In acetone atmosphere, the height of potential barriers decreases, leading to sensing signal. However, the response was not compared with metal oxide nanocomposites [105].
Metal-organic frameworks-derived zinc oxide nanopolyhedra/S, N: graphene quantum dots/polyaniline (ZnO/S, N: GQDs/PANI) nanohybrid was synthesized by in situ polymerization route for acetone sensing applications. The sensor was able to work at room temperature and a response (Ra/Rg) of 1.33–0.5 ppm acetone with a fast response time of 15 s was reported. The presence of PANI in the nanohybrid led to the formation of p–n heterojunctions and also caused to a redistribution of charge carriers at the interface of n-type ZnO and p-type PANI/S, N: GQDs, decreasing the activation energy needed for the adsorption of acetone gas molecules. The presence of S, N: GQDs in the nanohybrid created Schottky contacts, which further enhanced the sensor response through effective capture and migration of electrons [106].
PPy-WO3 hybrid nanocomposites with different weight percentages of WO3 NPs (5–40 wt%) dispersed in polypyrrole (PPy) matrix were prepared for acetone sensing studies. PPy-WO3(20 wt%) sensor showed a fast, fairly sensitive, selective, and enhanced response toward acetone at 90 °C. The enhancement of acetone sensing properties of the PPy-WO3 hybrid nanocomposite film was related to the effective role of WO3 NPs in PPy matrix and formation of p–n hetrojunction region. The results demonstrate the potential application of PPy-WO3 hybrid sensor for noninvasive detection of acetone in breath [107].
This entry is adapted from the peer-reviewed paper 10.3390/s20113096
References
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141.
- Cicolella, A. Volatile Organic Compounds (VOC): Definition, classification and properties. Rev. Mal. Respir. 2008, 25, 155–163.
- Zhang, D.; Fan, Y.; Li, G.; Du, W.; Li, R.; Liu, Y.; Cheng, Z.; Xu, J. Biomimetic synthesis of zeolitic imidazolate frameworks and their application in high performance acetone gas sensors. Sens. Actuators B Chem. 2020, 302, 127187.
- Morgott, D.A. Acetone. J. Patty’s Toxicol. 2001.
- Abdelghani, R.; Shokry Hassan, H.; Morsi, I.; Kashyout, A.B. Nano-architecture of highly sensitive SnO2–based gas sensors for acetone and ammonia using molecular imprinting technique. Sens. Actuators B Chem. 2019, 297, 126668.
- Yang, X.; Hao, X.; Liu, T.; Liu, F.; Wang, B.; Ma, C.; Liang, X.; Yang, C.; Zhu, H.; Zheng, J.; et al. CeO2-based mixed potential type acetone sensor using La1−xSrxCoO3 sensing electrode. Sens. Actuators B Chem. 2018, 269, 118–126.
- Šetka, M.; Bahos, F.A.; Matatagui, D.; Gràcia, I.; Figueras, E.; Drbohlavová, J.; Vallejos, S. Love wave sensors with silver modified polypyrrole nanoparticles for VOCs monitoring. Sensors 2020, 20, 1432.
- Baharuddin, A.A.; Ang, B.C.; Haseeb, A.S.M.A.; Wong, Y.C.; Wong, Y.H. Advances in chemiresistive sensors for acetone gas detection. Mater. Sci. Semicond. Process. 2019, 103, 104616.
- Masikini, M.; Chowdhury, M.; Nemraoui, O. Metal oxides: Application in exhaled breath acetone chemiresistive sensors. J. Electrochem. Soc. 2020, 167, 037537.
- Amann, A.; Poupart, G.; Telser, S.; Ledochowski, M.; Schmid, A.; Mechtcheriakov, S. Applications of breath gas analysis in medicine. Int. J. Mass Spectrom. 2004, 239, 227–233.
- Goto, T.; Itoh, T.; Akamatsu, T.; Sasaki, Y.; Sato, K.; Shin, W. Heat transfer control of micro-thermoelectric gas sensor for breath gas monitoring. Sens. Actuators B Chem. 2017, 249, 571–580.
- Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Schwarz, K.; Ligor, M.; Ligor, T.; Filipiak, W.; Denz, H.; Fiegl, M.; et al. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 2009, 9, 348.
- Kupari, M.; Lommi, J.; Ventilä, M.; Karjalainen, U. Breath acetone in congestive heart failure. Am. J. Cardiol. 1995, 76, 1076–1078.
- Chuang, M.-Y.; Lin, Y.-T.; Tung, T.-W.; Chang, L.-Y.; Zan, H.-W.; Meng, H.-F.; Lu, C.-J.; Tao, Y.-T. Room-temperature-operated organic-based acetone gas sensor for breath analysis. Sens. Actuators B Chem. 2018, 260, 593–600.
- Sujono, H.A.; Rivai, M.; Amin, M. Asthma identification using gas sensors and support vector machine. Telecommun. Comput. Electron. Control 2018, 16, 1468–1480.
- Nguyen, K.; Hung, C.M.; Ngoc, T.M.; Le, D.T.T.; Nguyen, D.H.; Van, D.N.; Van, H.N. Low-temperature prototype hydrogen sensors using Pd-decorated SnO2 nanowires for exhaled breath applications. Sens. Actuators B Chem. 2017, 253, 156–163.
- Chuang, M.-Y.; Chen, C.-C.; Zan, H.-W.; Meng, H.-F.; Lu, C.-J. Organic gas sensor with an improved lifetime for detecting breath ammonia in hemodialysis patients. ACS Sens. 2017, 2, 1788–1795.
- Zhang, S.; Jiang, W.; Li, Y.; Yang, X.; Sun, P.; Liu, F.; Yan, X.; Gao, Y.; Liang, X.; Ma, J.; et al. Highly-sensitivity acetone sensors based on spinel-type oxide (NiFe2O4) through optimization of porous structure. Sens. Actuators B Chem. 2019, 291, 266–274.
- Righettoni, M.; Tricoli, A. Toward portable breath acetone analysis for diabetes detection. J. Breath Res. 2011, 5, 037109.
- Teshima, N.; Li, J.; Toda, K.; Dasgupta, P.K. Determination of acetone in breath. Anal. Chim. Acta 2005, 535, 189–199.
- Deng, C.; Zhang, J.; Yu, X.; Zhang, W.; Zhang, X. Determination of acetone in human breath by gas chromatography–mass spectrometry and solid-phase microextraction with on-fiber derivatization. J. Chromatogr. B 2004, 810, 269–275.
- Sekine, Y.; Toyooka, S.; Watts, S.F. Determination of acetaldehyde and acetone emanating from human skin using a passive flux sampler—HPLC system. J. Chromatogr. B 2007, 859, 201–207.
- Wisthaler, A.; Jensen, N.; Winterhalter, R.; Lindinger, W.; Hjorth, J. Measurements of acetone and other gas phase product yields from the OH-initiated oxidation of terpenes by proton-transfer-reaction mass spectrometry (PTR-MS). J. Atmos. Environ. 2001, 35, 6181–6191.
- Mirzaei, A.; Kim, S.S.; Kim, H.W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances. J. Hazard. Mater. 2018, 357, 314–331.
- Ayesh, A.I. Linear hydrogen gas sensors based on bimetallic nanoclusters. J. Alloys Compd. 2016, 689, 1–5.
- Ayesh, A.I.; Karam, Z.; Awwad, F.; Meetani, M.A. Conductometric graphene sensors decorated with nanoclusters for selective detection of Hg2+ traces in water. Sens. Actuators B Chem. 2015, 221, 201–206.
- Vernieres, J.; Steinhauer, S.; Zhao, J.; Chapelle, A.; Menini, P.; Dufour, N.; Diaz, R.E.; Nordlund, K.; Djurabekova, F.; Grammatikopoulos, P. Gas phase synthesis of multifunctional Fe-based nanocubes. Adv. Funct. Mater. 2017, 27, 1605328.
- Jian, Y.; Hu, W.; Zhao, Z.; Cheng, P.; Haick, H.; Yao, M.; Wu, W. Gas sensors based on chemi-resistive hybrid functional nanomaterials. Nano Micro Lett. 2020, 12, 1–43.
- Kim, S.J.; Koh, H.-J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.-Y.; Anasori, B.; Kim, C.-K.; Choi, Y.-K.; Kim, J. Metallic Ti3C2Tx MXene gas sensors with ultrahigh signal-to-noise ratio. ACS Nano 2018, 12, 986–993.
- Park, H.J.; Choi, N.-J.; Kang, H.; Jung, M.Y.; Park, J.W.; Park, K.H.; Lee, D.-S. A ppb-level formaldehyde gas sensor based on CuO nanocubes prepared using a polyol process. Sens. Actuators B Chem. 2014, 203, 282–288.
- Choi, M.S.; Bang, J.H.; Mirzaei, A.; Oum, W.; Na, H.G.; Jin, C.; Kim, S.S.; Kim, H.W. Promotional effects of ZnO-branching and Au-functionalization on the surface of SnO2 nanowires for NO2 sensing. J. Alloys Compd. 2019, 786, 27–39.
- Kao, K.-W.; Hsu, M.-C.; Chang, Y.-H.; Gwo, S.; Yeh, J.A. A sub-ppm acetone gas sensor for diabetes detection using 10 nm thick ultrathin InN FETs. Sensors 2012, 12, 7157–7168.
- Subashini, T.; Renganathan, B.; Stephen, A.; Prakash, T. Acetone sensing behaviour of optical fiber clad-modified with γ-CuBr nanocrystals. Mater. Sci. Semicond. Process. 2018, 88, 181–185.
- Aparicio-Martínez, E.; Osuna, V.; Dominguez, R.B.; Márquez-Lucero, A.; Zaragoza-Contreras, E.A.; Vega-Rios, A. Room temperature detection of acetone by a PANI/cellulose/WO3 electrochemical sensor. J. Nanomater. 2018, 2018, 6519694.
- Zhang, C.; Ghosh, A.; Zhang, H.; Shi, S.Q. Langasite-based surface acoustic wave resonator for acetone vapor sensing. Smart Mater. Struct. 2019, 29, 015039.
- Huang, X.; Huang, Z.; Zhang, L.; Liu, R.; Lv, Y. Highly efficient cataluminescence gas sensor for acetone vapor based on UIO-66 metal-organic frameworks as preconcentrator. Sens. Actuators B Chem. 2020, 312, 127952.
- Staszek, K.; Szkudlarek, A.; Kawa, M.; Rydosz, A. Microwave system with sensor utilizing GO-based gas-sensitive layer and its application to acetone detection. Sens. Actuators B Chem. 2019, 297, 126699.
- Grall, S.; Debéda, H.; Dufour, I.; Aubry, V. Screen-printed microcantilevers for environmental sensing. In Proceedings of the EUROSENSORS 2018 Conference, Graz, Austria, 9–12 September 2018; p. 722.
- da Silva, L.F.; Catto, A.C.; Avansi, W.; Cavalcante, L.S.; Mastelaro, V.R.; Andrés, J.; Aguir, K.; Longo, E. Acetone gas sensor based on α-Ag2WO4 nanorods obtained via a microwave-assisted hydrothermal route. J. Alloys Compd. 2016, 683, 186–190.
- Hao, X.; Wu, D.; Wang, Y.; Ouyang, J.; Wang, J.; Liu, T.; Liang, X.; Zhang, C.; Liu, F.; Yan, X.; et al. Gas sniffer (YSZ-based electrochemical gas phase sensor) toward acetone detection. Sens. Actuators B Chem. 2019, 278, 1–7.
- Jin, T.; Zhou, J.; Lin, P.T. Mid-infrared waveguides for volatile organic compounds detection. In Proceedings of the Optics and Photonics for Sensing the Environment, San Jose, CA, USA, 25–27 June 2019; p. EW2A.2.
- Sabri, Y.M.; Kandjani, A.E.; Rashid, S.S.A.A.H.; Harrison, C.J.; Ippolito, S.J.; Bhargava, S.K. Soot template TiO2 fractals as a photoactive gas sensor for acetone detection. Sens. Actuators B Chem. 2018, 275, 215–222.
- Wang, S.; Wang, L.; Yang, T.; Liu, X.; Zhang, J.; Zhu, B.; Zhang, S.; Huang, W.; Wu, S. Porous α-Fe2O3 hollow microspheres and their application for acetone sensor. J. Solid State Chem. 2010, 183, 2869–2876.
- Lalauze, R. Chemical Sensors and Biosensors; John Wiley & Sons: Hoboken, NJ, USA, 2012.
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Neri, G. oxide-shell nanomaterials for gas-sensing applications: A review. J. Nanopart. Res. 2015, 17, 371.
- Briand, D.; Courbat, J. Micromachined semiconductor gas sensors. In Semiconductor Gas Sensors; Elsevier: Amsterdam, The Netherlands, 2020; pp. 413–464.
- Ayesh, A.I.; Ahmed, R.E.; Al-Rashid, M.A.; Alarrouqi, R.A.; Saleh, B.; Abdulrehman, T.; Haik, Y.; Al-Sulaiti, L.A. Selective gas sensors using graphene and CuO nanorods. Sens. Actuators A Phys. 2018, 283, 107–112.
- Galstyan, V.; Ponzoni, A.; Kholmanov, I.; Natile, M.M.; Comini, E.; Sberveglieri, G. Highly sensitive and selective detection of dimethylamine through Nb-doping of TiO2 nanotubes for potential use in seafood quality control. Sens. Actuators B Chem. 2020, 303, 127217.
- Capone, S.; Benkovicova, M.; Forleo, A.; Jergel, M.; Manera, M.G.; Siffalovic, P.; Taurino, A.; Majkova, E.; Siciliano, P.; Vavra, I.; et al. Palladium/γ-Fe2O3 nanoparticle mixtures for acetone and NO2 gas sensors. Sens. Actuators B Chem. 2017, 243, 895–903.
- Yang, H.M.; Ma, S.Y.; Jiao, H.Y.; Chen, Q.; Lu, Y.; Jin, W.X.; Li, W.Q.; Wang, T.T.; Jiang, X.H.; Qiang, Z.; et al. Synthesis of Zn2SnO4 hollow spheres by a template route for high-performance acetone gas sensor. Sens. Actuators B Chem. 2017, 245, 493–506.
- Kim, S.; Park, S.; Sun, G.-J.; Hyun, S.K.; Kim, K.-K.; Lee, C. Enhanced acetone gas sensing performance of the multiple-networked Fe2O3-functionalized In2O3 nanowire sensor. Curr. Appl. Phys. 2015, 15, 947–952.
- Ge, M.; Xuan, T.; Yin, G.; Lu, J.; He, D. Controllable synthesis of hierarchical assembled porous ZnO microspheres for acetone gas sensor. Sens. Actuators B Chem. 2015, 220, 356–361.
- Li, X.B.; Ma, S.Y.; Li, F.M.; Chen, Y.; Zhang, Q.Q.; Yang, X.H.; Wang, C.Y.; Zhu, J. Porous spheres-like ZnO nanostructure as sensitive gas sensors for acetone detection. Mater. Lett. 2013, 100, 119–123.
- Agarwal, S.; Rai, P.; Gatell, E.N.; Llobet, E.; Güell, F.; Kumar, M.; Awasthi, K. Gas sensing properties of ZnO nanostructures (flowers/rods) synthesized by hydrothermal method. Sens. Actuators B Chem. 2019, 292, 24–31.
- Qi, Q.; Zhang, T.; Liu, L.; Zheng, X.; Yu, Q.; Zeng, Y.; Yang, H. Selective acetone sensor based on dumbbell-like ZnO with rapid response and recovery. Sens. Actuators B Chem. 2008, 134, 166–170.
- Kim, H.W.; Kwon, Y.J.; Mirzaei, A.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Kim, S.S. Synthesis of zinc oxide semiconductors-graphene nanocomposites by microwave irradiation for application to gas sensors. Sens. Actuators B Chem. 2017, 249, 590–601.
- Jiao, W.-l.; Zhang, L. Preparation and gas sensing properties for acetone of amorphous Ag modified NiFe2O4 sensor. Trans. Nonferr. Met. Soc. China 2012, 22, 1127–1132.
- Gurlo, A. Interplay between O2 and SnO2: Oxygen ionosorption and spectroscopic evidence for adsorbed oxygen. J. Chemphyschem. 2006, 7, 2041–2052.
- Sahm, T.; Gurlo, A.; Barsan, N.; Weimar, U. Basics of oxygen and SnO2 interaction; work function change and conductivity measurements. Sens. Actuators B Chem. 2006, 118, 78–83.
- Wang, J.; Yang, J.; Han, N.; Zhou, X.; Gong, S.; Yang, J.; Hu, P.; Chen, Y. Highly sensitive and selective ethanol and acetone gas sensors based on modified ZnO nanomaterials. Mater. Des. 2017, 121, 69–76.
- Wang, P.; Dong, T.; Jia, C.; Yang, P. Ultraselective acetone-gas sensor based ZnO flowers functionalized by Au nanoparticle loading on certain facet. Sens. Actuators B Chem. 2019, 288, 1–11.
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. How shell thickness can affect the gas sensing properties of nanostructured materials: Survey of literature. Sens. Actuators B Chem. 2018, 258, 270–294.
- Poghossian, A.; Abovian, H.; Avakian, P.; Mkrtchian, S.; Haroutunian, V.J. Bismuth ferrites: New materials for semiconductor gas sensors. Sens. Actuators B Chem. 1991, 4, 545–549.
- Chakraborty, S.; Pal, M. Highly selective and stable acetone sensor based on chemically prepared bismuth ferrite nanoparticles. J. Alloys Compd. 2019, 787, 1204–1211.
- Ma, L.; Ma, S.Y.; Shen, X.F.; Wang, T.T.; Jiang, X.H.; Chen, Q.; Qiang, Z.; Yang, H.M.; Chen, H. PrFeO3 hollow nanofibers as a highly efficient gas sensor for acetone detection. Sens. Actuators B Chem. 2018, 255, 2546–2554.
- Zhou, X.; Liu, J.; Wang, C.; Sun, P.; Hu, X.; Li, X.; Shimanoe, K.; Yamazoe, N.; Lu, G. Highly sensitive acetone gas sensor based on porous ZnFe2O4 nanospheres. Sens. Actuators B Chem. 2015, 206, 577–583.
- Haija, M.A.; Basina, G.; Banat, F.; Ayesh, A.I. Adsorption and gas sensing properties of CuFe2O4 nanoparticles. Mater. Sci. Pol. 2019, 37, 289–295.
- Haija, M.A.; Abu-Hani, A.F.; Hamdan, N.; Stephen, S.; Ayesh, A.I. Characterization of H2S gas sensor based on CuFe2O4 nanoparticles. J. Alloys Compd. 2017, 690, 461–468.
- Haija, M.A.; Ayesh, A.I.; Ahmed, S.; Katsiotis, M.S. Selective hydrogen gas sensor using CuFe2O4 nanoparticle based thin film. Appl. Surf. Sci. 2016, 369, 443–447.
- Šutka, A.; Gross, K.A. Spinel ferrite oxide semiconductor gas sensors. Sens. Actuators B Chem. 2016, 222, 95–105.
- Ayesh, A.I.; Abu Haija, M.; Shaheen, A.; Banat, F. Spinel ferrite nanoparticles for H2S gas sensor. Appl. Phys. A 2017, 123, 682.
- Abu-Hani, A.F.S.; Mahmoud, S.T.; Awwad, F.; Ayesh, A.I. Design, fabrication, and characterization of portable gas sensors based on spinel ferrite nanoparticles embedded in organic membranes. Sens. Actuators B Chem. 2017, 241, 1179–1187.
- Lv, L.; Wang, Y.; Cheng, P.; Zhang, B.; Dang, F.; Xu, L. Ultrasonic spray pyrolysis synthesis of three-dimensional ZnFe2O4-based macroporous spheres for excellent sensitive acetone gas sensor. Sens. Actuators B Chem. 2019, 297, 126755.
- Li, L.; Tan, J.; Dun, M.; Huang, X. Porous ZnFe2O4 nanorods with net-worked nanostructure for highly sensor response and fast response acetone gas sensor. Sens. Actuators B Chem. 2017, 248, 85–91.
- Patil, J.Y.; Nadargi, D.Y.; Mulla, I.S.; Suryavanshi, S.S. Cerium doped MgFe2O4 nanocomposites: Highly sensitive and fast response-recoverable acetone gas sensor. Heliyon 2019, 5, e01489.
- Khandekar, M.S.; Tarwal, N.L.; Mulla, I.S.; Suryavanshi, S.S. Nanocrystalline Ce doped CoFe2O4 as an acetone gas sensor. Ceram. Int. 2014, 40, 447–452.
- Wang, C.; Liu, J.; Yang, Q.; Sun, P.; Gao, Y.; Liu, F.; Zheng, J.; Lu, G. Ultrasensitive and low detection limit of acetone gas sensor based on W-doped NiO hierarchical nanostructure. Sens. Actuators B Chem. 2015, 220, 59–67.
- Al-Hadeethi, Y.; Umar, A.; Al-Heniti, S.H.; Kumar, R.; Kim, S.H.; Zhang, X.; Raffah, B.M. 2D Sn-doped ZnO ultrathin nanosheet networks for enhanced acetone gas sensing application. Ceram. Int. 2017, 43, 2418–2423.
- Xu, X.L.; Chen, Y.; Ma, S.Y.; Li, W.Q.; Mao, Y.Z. Excellent acetone sensor of La-doped ZnO nanofibers with unique bead-like structures. Sens. Actuators B Chem. 2015, 213, 222–233.
- Liu, L.; Li, S.; Zhuang, J.; Wang, L.; Zhang, J.; Li, H.; Liu, Z.; Han, Y.; Jiang, X.; Zhang, P. Improved selective acetone sensing properties of Co-doped ZnO nanofibers by electrospinning. Sens. Actuators B Chem. 2011, 155, 782–788.
- Cheng, J.P.; Wang, B.B.; Zhao, M.G.; Liu, F.; Zhang, X.B. Nickel-doped tin oxide hollow nanofibers prepared by electrospinning for acetone sensing. Sens. Actuators B Chem. 2014, 190, 78–85.
- Jiang, Z.; Zhao, R.; Sun, B.; Nie, G.; Ji, H.; Lei, J.; Wang, C. Highly sensitive acetone sensor based on Eu-doped SnO2 electrospun nanofibers. Ceram. Int. 2016, 42, 15881–15888.
- Ulyanov, A.N.; Sidorov, A.V.; Pismenova, N.E.; Goodilin, E.A.; Savilov, S.V. Self-doped La1−xMnO3+δ perovskites: Electron state hybridization and Raman modes. Solid State Sci. 2019, 94, 41–44.
- Liu, H.; Li, C.; Zhang, X.; Zheng, K.; Xie, R.; Huang, H.; Peng, T.; Jia, R.; Huo, J. A novel and highly responsive acetone sensor based on La1−xYxMnO3+δ nanoparticles. Mater. Lett. 2019, 257, 126725.
- Zhang, P.; Qin, H.; Lv, W.; Zhang, H.; Hu, J. Gas sensors based on ytterbium ferrites nanocrystalline powders for detecting acetone with low concentrations. Sens. Actuators B Chem. 2017, 246, 9–19.
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75.
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255.
- Zhang, Y.; Zhou, L.; Liu, Y.; Liu, D.; Liu, F.; Liu, F.; Yan, X.; Liang, X.; Gao, Y.; Lu, G. Gas sensor based on samarium oxide loaded mulberry-shaped tin oxide for highly selective and sub ppm-level acetone detection. J. Colloid Interface Sci. 2018, 531, 74–82.
- Park, S. Acetone gas detection using TiO2 nanoparticles functionalized In2O3 nanowires for diagnosis of diabetes. J. Alloys Compd. 2017, 696, 655–662.
- Kim, N.-H.; Choi, S.-J.; Kim, S.-J.; Cho, H.-J.; Jang, J.-S.; Koo, W.-T.; Kim, M.; Kim, I.-D. Highly sensitive and selective acetone sensing performance of WO3 nanofibers functionalized by Rh2O3 nanoparticles. Sens. Actuators B Chem. 2016, 224, 185–192.
- Wang, X.-J.; Wang, W.; Liu, Y.-L. Enhanced acetone sensing performance of Au nanoparticles functionalized flower-like ZnO. Sens. Actuators B Chem. 2012, 168, 39–45.
- Wongrat, E.; Chanlek, N.; Chueaiarrom, C.; Thupthimchun, W.; Samransuksamer, B.; Choopun, S. Acetone gas sensors based on ZnO nanostructures decorated with Pt and Nb. Ceram. Int. 2017, 43, S557–S566.
- Zhang, S.; Yang, M.; Liang, K.; Turak, A.; Zhang, B.; Meng, D.; Wang, C.; Qu, F.; Cheng, W.; Yang, M. An acetone gas sensor based on nanosized Pt-loaded Fe2O3 nanocubes. Sens. Actuators B Chem. 2019, 290, 59–67.
- Liu, W.; Xu, L.; Sheng, K.; Zhou, X.; Dong, B.; Lu, G.; Song, H. A highly sensitive and moisture-resistant gas sensor for diabetes diagnosis with 2O3 nanowires and a molecular sieve for protection. NPG Asia Mater. 2018, 10, 293–308.
- Huang, Z.F.; Song, J.; Pan, L.; Zhang, X.; Wang, L.; Zou, J.J. Tungsten oxides for photocatalysis, electrochemistry, and phototherapy. Adv. Mater. 2015, 27, 5309–5327.
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331.
- Sun, S.; Wang, M.; Chang, X.; Jiang, Y.; Zhang, D.; Wang, D.; Zhang, Y.; Lei, Y. W18O49/Ti3C2Tx Mxene nanocomposites for highly sensitive acetone gas sensor with low detection limit. Sens. Actuators B Chem. 2020, 304, 127274.
- Ayesh, A.I.; Abu-Hani, A.F.S.; Mahmoud, S.T.; Haik, Y. Selective H2S sensor based on CuO nanoparticles embedded in organic membranes. Sens. Actuators B Chem. 2016, 231, 593–600.
- Siebert, L.; Wolff, N.; Ababii, N.; Terasa, M.-I.; Lupan, O.; Vahl, A.; Duppel, V.; Qiu, H.; Tienken, M.; Mirabelli, M.; et al. Facile fabrication of semiconducting oxide nanostructures by direct ink writing of readily available metal microparticles and their application as low power acetone gas sensors. Nano Energy 2020, 70, 104420.
- Zhang, L.; Dong, B.; Xu, L.; Zhang, X.; Chen, J.; Sun, X.; Xu, H.; Zhang, T.; Bai, X.; Zhang, S.; et al. Three-dimensional ordered ZnO–Fe3O4 inverse opal gas sensor toward trace concentration acetone detection. Sens. Actuators B Chem. 2017, 252, 367–374.
- Hu, J.; Zou, C.; Su, Y.; Li, M.; Yang, Z.; Ge, M.; Zhang, Y. One-step synthesis of 2D C3N4-tin oxide gas sensors for enhanced acetone vapor detection. Sens. Actuators B Chem. 2017, 253, 641–651.
- Wang, L.; Teleki, A.; Pratsinis, S.E.; Gouma, P.I. Ferroelectric WO3 nanoparticles for acetone selective detection. Chem. Mater. 2008, 20, 4794–4796.
- Wang, P.; Wang, D.; Zhang, M.; Zhu, Y.; Xu, Y.; Ma, X.; Wang, X. ZnO nanosheets/graphene oxide nanocomposites for highly effective acetone vapor detection. Sens. Actuators B Chem. 2016, 230, 477–484.
- Huang, J.; Yang, T.; Kang, Y.; Wang, Y.; Wang, S. Gas sensing performance of polyaniline/ZnO organic-inorganic hybrids for detecting VOCs at low temperature. J. Nat. Gas Chem. 2011, 20, 515–519.
- Geng, L.; Zhao, Y.; Huang, X.; Wang, S.; Zhang, S.; Wu, S. Characterization and gas sensitivity study of polyaniline/SnO2 hybrid material prepared by hydrothermal route. Sens. Actuators B Chem. 2007, 120, 568–572.
- Zhang, D.; Wu, Z.; Zong, X. Metal-organic frameworks-derived zinc oxide nanopolyhedra/S, N: Graphene quantum dots/polyaniline ternary nanohybrid for high-performance acetone sensing. Sens. Actuators B Chem. 2019, 288, 232–242.
- Jamalabadi, H.; Alizadeh, N. Enhanced low-temperature response of PPy-WO3 hybrid nanocomposite based gas sensor deposited by electrospinning method for selective and sensitive acetone detection. IEEE Sens. J. 2017, 17, 2322–2328.
This entry is offline, you can click here to edit this entry!
