Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Heavy metals, known for their toxic nature and ability to accumulate and magnify in the food chain, are a major environmental concern. The use of environmentally friendly adsorbents, such as chitosan (CS)—a biodegradable cationic polysaccharide, has gained attention for removing heavy metals from water.
- adsorption
- chelation
- chitosan
- deacetylation
- heavy metals
1. Introduction
Various materials, including natural contaminants, fluoride, chloride, nitrate, iron, calcium, magnesium, and other contaminants from byproducts of agriculture and industries such as heavy metals, organic dyes, insecticides and fertilizers, spilled oils, batteries, diesel fuel, household chemicals such as synthetic detergents, and some pathogenic microbes can contaminate the ocean, groundwater and surface water bodies (rivers, lakes, ponds, reservoirs) [1][2][3][4][5][6]. Contaminated water will not be suitable for drinking, habitat, irrigation, recreation, and other industrial activities [4][7].
Heavy metal has gained significant importance in ecotoxicology due to their long persistence, bioaccumulation, and bio-magnification in the food chain [8]. Heavy metal ions are commonly discharged by various industrial activities, including electroplating, battery manufacturing, pesticide production, mining, nuclear power, textile manufacturing, and other similar industries [9][10][11]. The global average content of Cr, Mn, Co, Ni, As and Cd on surface water bodies exceeded the permitted level suggested by WHO and USEPA Guidelines [8]. Heavy metal ions, such as As, Cr, Ni, Co, Hg, Pb, U, and Cd, have become a major threat as they are toxic in nature and accumulate in food chains due to their non-biodegradability and cause various health-related issues even at trace levels. Therefore, in order to safeguard human health and the environment, it is crucial to eliminate heavy metals prior to their release into the surroundings [10][12][13][14][15].
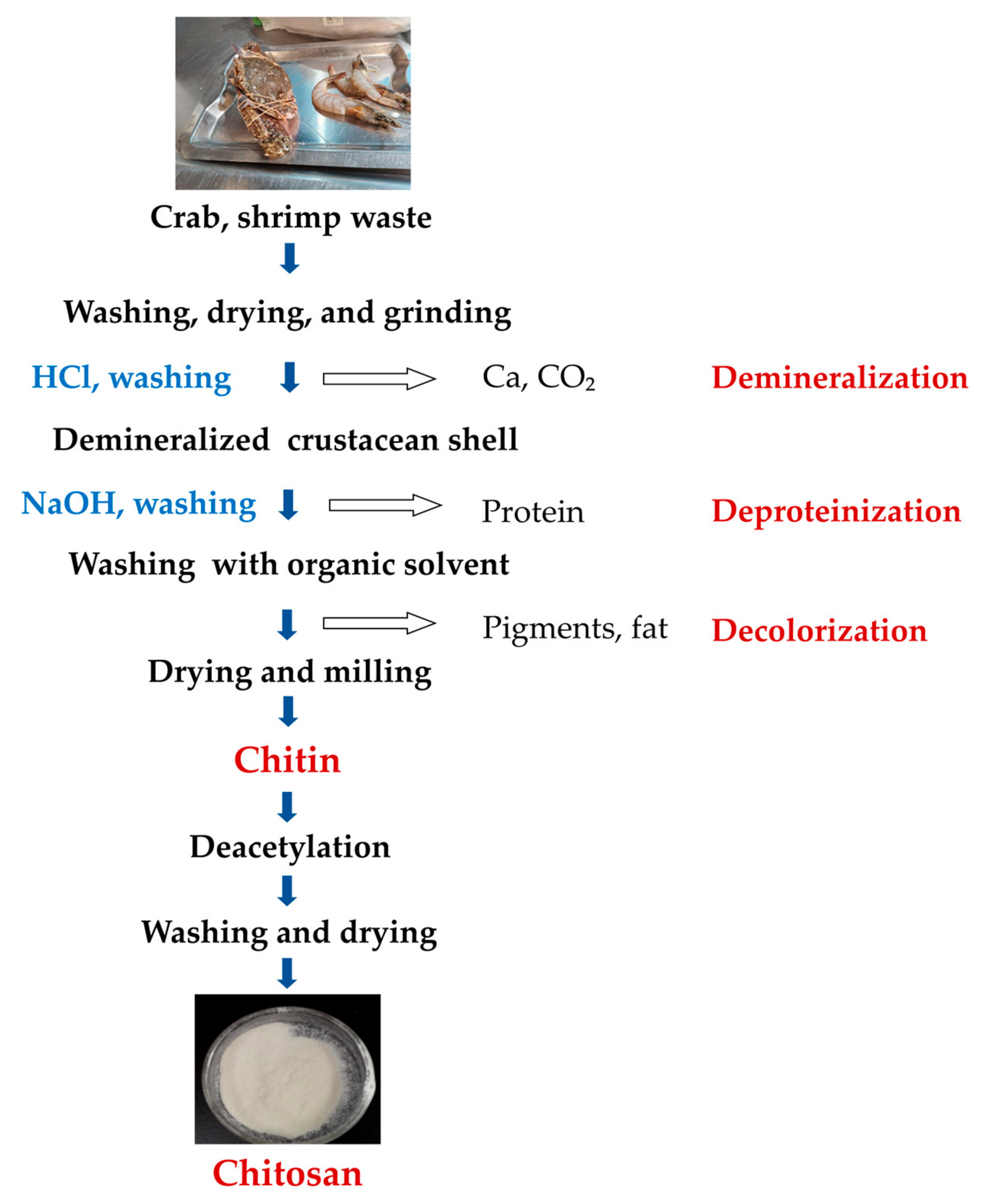
2. Chitin Sources and Composition
To address environmental concerns, the industrial production of chitin and chitosan (CS) must be carried out on a large scale and at a competitive cost [16]. Chitin can be found in various sources, including crustacean exoskeletons (lobsters, shrimps, prawns, crabs, krill, crayfish), mollusks (octopus, cuttlefish, clams, oysters, squids, snails), algae (diatoms, brown algae, green algae), insects cuticles, and fungal cell walls [17]. Presently, crustacean waste, such as shrimp, crabs, prawns, and lobsters, is the primary source of industrial chitin [17][18]. Though, chitin/chitosan from crustaceans has limitations, such as limited raw material supply, a seasonal, higher concentration of CaCO3, requirement of chemical treatment, the possibility of heavy metal contamination, and consume long time. In contrast, chitin/chitosan fungal sources have seasonal independence, superior particle size, uniformity, lower molecular weight, and are free from heavy metals [19]. Table 1 outlines the proximate composition of several chitin sources, with shells comprising chitin, protein, minerals, and pigments [20]. Crustacean (crab, shrimp, and lobster) shell wastes consist of 15–40% chitin, 20–50% CaCO3, and 20–40% protein, along with lipids, pigments, and other minerals in small amounts [21]. Therefore, removing proteins, minerals, and pigments is necessary for chitin production [18].
Table 1. Proximate composition of different chitin sources [22].
| Sources | Protein (%) | Ash (%) | Chitin (%) | Moisture (%) | Lipid (%) |
|---|---|---|---|---|---|
| Shrimp shells | 32.77 | 32.46 | 36.43 | 45.65 | - |
| Shrimp shells (P. longirostris) |
29.23 | 25.06 | 26.98 | 3.25 | 15.48 |
| Shrimp shells (Penaeus durarum) |
34.02 | 42.26 | 23.72 | - | - |
| Insect cuticles (Cicada sloughs) |
39.8 | 11.7 | 36.6 | 8.7 | 2.7 |
| Crabs’ shells | 16.68 | 66.58 | 16.73 | - | - |
| Mussel shells | 9.99 | 23.25 | 23.25 | - | - |
| Squid gladius (L. vulgaris) |
36.52 | 2.57 | 31.2 | - | 0..32 |
Adapted from [22].
3. Production of Chitosan from Chitin
The chitin production process can be achieved via four main steps: sample preparation, demineralization (removal of the mineral fraction, mainly CaCO3), deproteinization (removal of protein fraction), and discoloration (removal of carotenoid pigments) (Figure 1). In order to obtain pure chitin without impurities, demineralization, deproteinization, and decolorization are necessary. Demineralization is achieved using acid and deproteinization using alkali, while decolorizing agents such as chloroform, hydrogen peroxide, and acetone are used to remove pigments. It is important to correctly manipulate time, chemical concentration, and temperature to achieve the highest molecular weight of chitin/CS [23]. The deproteinization and demineralization steps can be carried out in reverse order [24], and CS can be produced by deacetylating chitin. A flow diagram for the isolation of chitin and the production of CS is shown in Figure 1.

Figure 1. A simplified flow diagram of chitin and CS preparation [24][25].
4. Characteristics of Chitosan
4.1. Molecular Weight
The N-deacetylation reaction results in molecular weight reduction [26]. Natural chitin typically has a molecular weight exceeding 1000 kDa. Commercially available CS usually ranges from 100–1000 kDa, and its molecular weight is affected by the preparation method and the source of raw materials [27][28]. For example, the molecular weight of CS from insects has reported lower values, ranging from 26–300 kDa [29]. Degradation of CS can be caused by processing factors such as elevated temperature, dissolved oxygen, and shear stress. High temperature or concentrated acids (e.g., HCl, CH3COOH) cause molecular weight changes, while EDTA results in minimal degradation [30]. Techniques such as HPLC and light scattering methods can be used to determine the molecular weight distributions of CS [26].
4.2. Degree of Deacetylation
Chitin is deacetylated by removing the acetyl group, resulting in amino groups (–NH2) that are highly chemically reactive [30]. The degree of deacetylation is a crucial determinant of the physicochemical properties and biodegradation of the material. When the degree of deacetylation of chitin is 50% or higher, it is generally known as CS [31]. The degree of deacetylation is the proportion of glucosamine monomers in the chitosan chain and can affect the solubility and performance of chitosan in various applications [29]. The degree of deacetylation can be determined using different tools, such as infrared (IR) spectroscopy or nuclear magnetic resonance (NMR) spectroscopy [23][26][29].
4.3. Solubility
The strong intra- and intermolecular hydrogen bonding and highly crystalline structure of chitin make it insoluble in water, dilute aqueous salt solutions, and most organic solvents [26][32]. In contrast, CS is a cationic polysaccharide soluble in dilute aqueous acidic solution (below pH 6) [31][32]. Protonation plays an important role in the solubility of CS in acids, such as CH3COOH and HCl, and the pH and the pK of the acid determine the degree of ionization on -NH2 functional group at C-2 of the d-glucosamine repeat unit [33].
Along with the pH, the ionic strength of the solvent determines the degree of protonation or charge density of the amines group, thereby, the solubility of CS. CS is soluble in acidic pH (<pH 6) and insoluble in neutral and basic pH [24]. Organic acids such as acetic, formic, and lactic acids are used for dissolving CS [30].
The solubility and applicability of CS depend on two primary factors: the degree of deacetylation and molecular weight. When the degree of acetylation is below 50%, CS obtained from partially deacetylated chitin can dissolve in acidic conditions [23].
The low deacetylated degree of CS (deacetylation degree of 55–70%) is almost completely insoluble in water. Whereas CS with the middle deacetylation degree (70–85%) is partly dissolved in water, and the high deacetylation degree (85–95%) of CS has good solubility in water. Moreover, the ultrahigh deacetylation degree of CS (95–100%) is difficult to obtain [34].
5. Neutralization and Chemical and Physical Modification of Chitosan
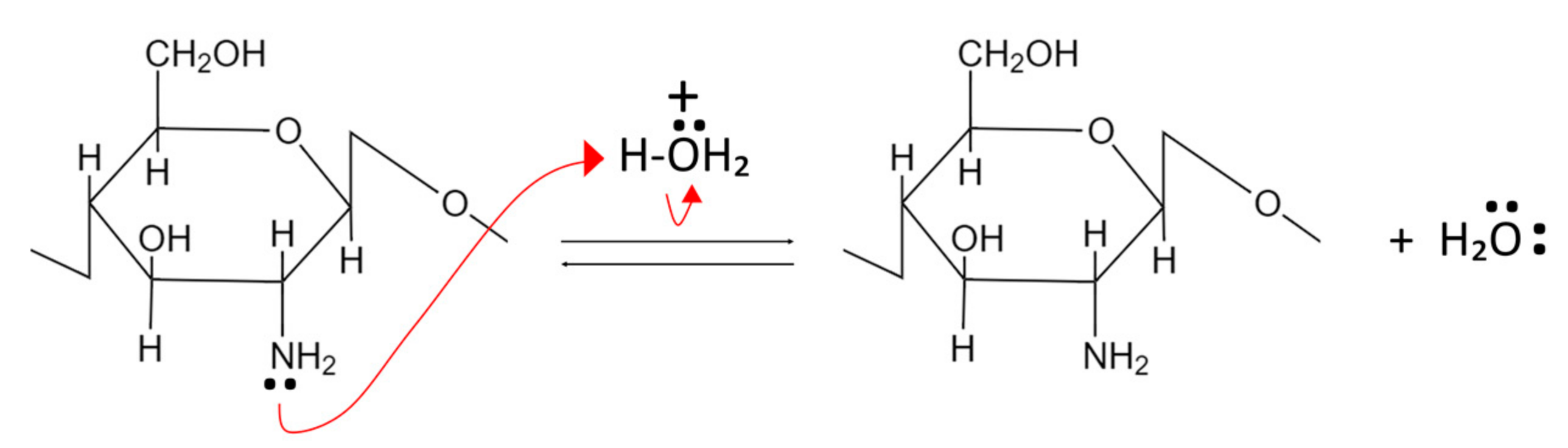
Chitin and CS act as weak bases and undergo alkaline compound neutralization reactions. In this process, the primary amine group’s nonbonding pair of electrons from the glucosamine units accept protons and become positively charged, as shown in Figure 2 [35][36].

Figure 2. Neutralization reaction of chitin and CS.
CS is not soluble at neutral and basic pH but can dissolve in acidic pH due to increased polarity and polymer-polymer electrostatic repulsion. In contrast, chitin remains insoluble even in acidic pH because it has fewer amino groups than CS [35].
Due to the presence of a non-bonding electron pair in the primary amino groups, CS functions as a potent nucleophile. Additionally, the primary amine groups at CS exhibit greater nucleophilic characteristics than the primary hydroxyl groups found at the C-6 position [35][36].
Due to the presence of the larger amount of amino (at C-2) and hydroxyl groups (at C-3 and C-6), CS can undergo various chemical modifications, such as acylation, carboxylation and etherification, hydroxylation, phosphorylation, thiolation, methylation, sulphonation, quaternization, crosslinking, graft copolymerization, chelation, oxidation, alkylation etc. This chemical modification generates various CS derivatives with improved solubility (in water or organic solvents), as well as other physicochemical and mechanical properties, which make them a potential material in many applications in various fields [32][36][37].
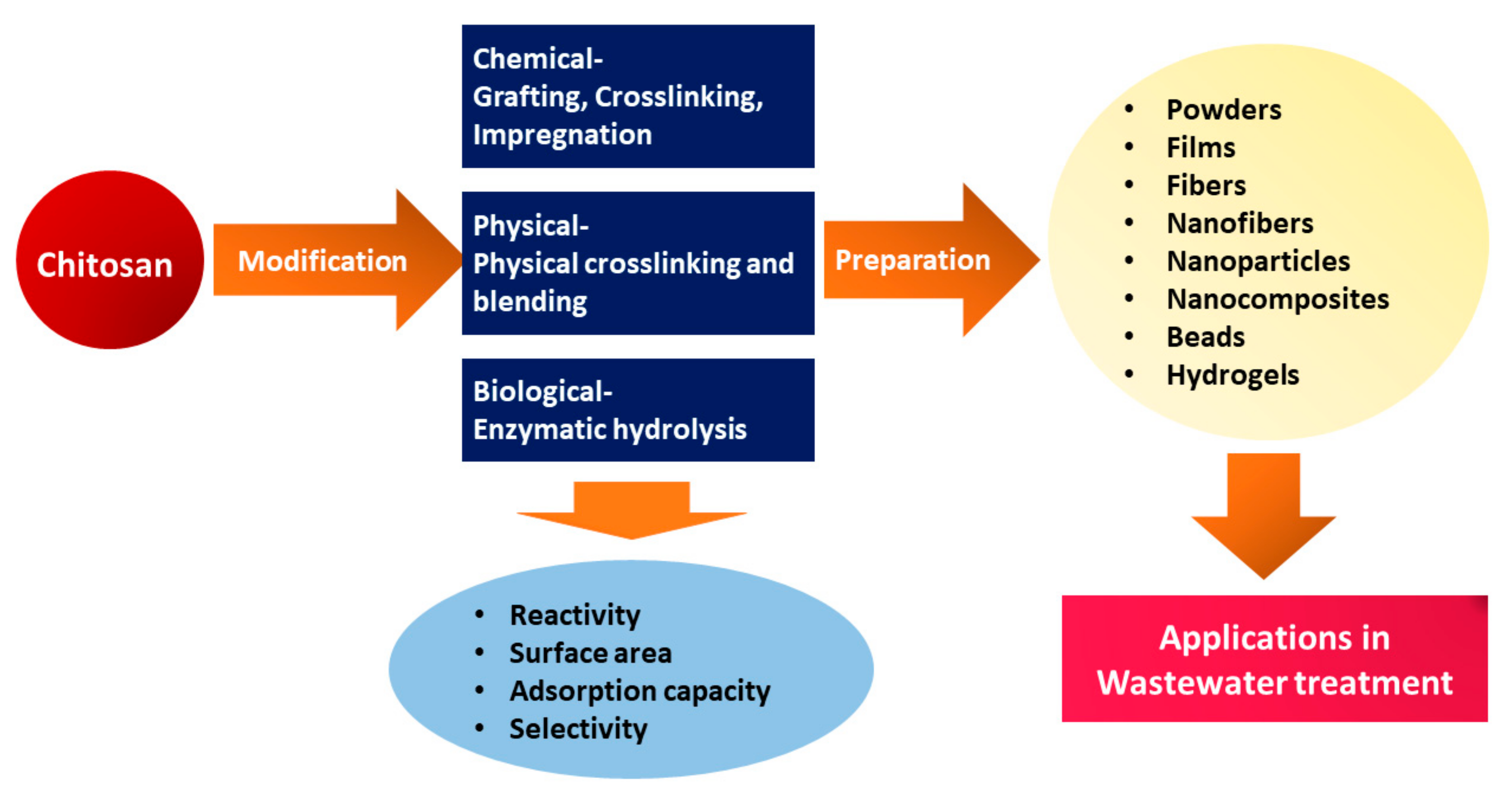
Furthermore, CS can be modified through various physical modifications into different forms, including solution, powder, flake, fiber, nanofiber, beads, and film, allowing for a wide range of applications across multiple fields, including water treatment (Figure 3) [38][39]. Moreover, to improve adsorption performance, CS has been modified into various forms, such as hydrogel, nanoparticles, and nanofibers, due to low specific area and porosity in powder and flake forms [40].

Figure 3. Schematic representation of modification of CS and CS-based materials preparation for wastewater treatment.
6. Waste Treatment and Purification of Water
6.1. Chitosan Based Composites to Eliminate Heavy Metal Ions
The non-biodegradability, carcinogenicity, and toxicity at low concentrations make organic dyes and heavy metals the most dangerous water pollutants [41]. Heavy metals, in particular, pose a significant risk to both human health and the environment as they are toxic and can accumulate and magnify in the food chain [12]. To ensure safe water consumption and human activities, it is crucial to eliminate heavy metals from the water before discharging them into the environment [12][41].
To remediate water, different techniques have been employed, such as flotation, coagulation/flocculation, oxidation-reduction, precipitation, membrane techniques (nanofiltration, ultrafiltration, reverse osmosis), ion exchange, electrochemical, photocatalytic degradation, and adsorption [27][42]. Among these methods, adsorption has a high removal rate, easy accessibility, low cost, and reduced secondary pollution [27][43]. Various parameters, such as pH, temperature, contact time, adsorbent dosage, initial metal concentration, and type of sorbent (surface area, porosity, functional group distribution), influence the metal adsorption process [44][45].
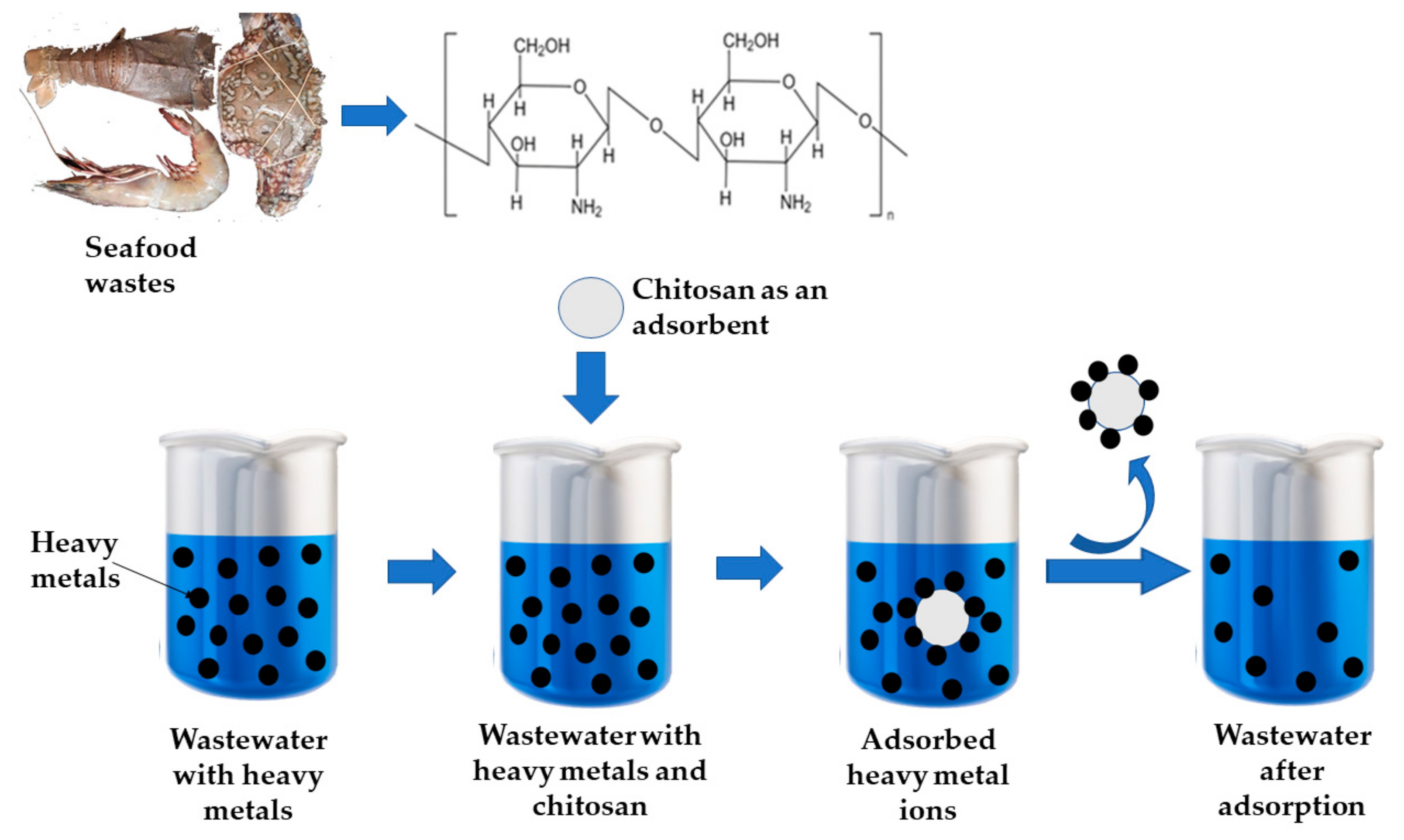
Water treatment for heavy metal (As, Pb, Hg, Cd, Cu, Cr) and radionuclide (U, Se, Tc) removal has been accomplished using water-stable adsorbents made from metal-organic frameworks (MOF) and MOF-based composites. These materials possess remarkable porosity and surface area, facilitating adsorption and/or photocatalytic redox processes [46]. Figure 4 depicts CS’s ability to remove heavy metals from wastewater.

Figure 4. Chitosan is employed for removing heavy metals from wastewater.
6.2. Chitosan Based Nanocomposites to Eliminate Heavy Metal Ions
The outstanding properties of nanomaterials, including high surface areas, numerous active sites, and exceptional sorption capabilities, make them highly valuable for treating wastewater [47][48][49]. In recent years, numerous studies have focused on producing magnetic CS-based nanocomposites for extracting metal ions [50][51][52]. Nano-absorbents made from magnetic CS nanoparticles or nanocomposites have been investigated for their effectiveness in removing metal ions from wastewater. The CS polymer chain contains an increased number of hydroxyl and amino groups, which make it highly selective and capable of strongly chelating metals, enabling efficient sorbent regeneration.
To enhance the mechanical properties of composite materials, a green and efficient approach involves adding different reinforcing fillers to the polymer matrix [53]. The SiO2/CS composite is capable of adsorbing heavy metal ions in solution, particularly As(V) and Hg(II), with a high performance (198.6 and 204.1 mg/g, respectively). SiO2 possesses stable chemical properties and good mechanical strength and can effectively prevent the adsorbents from agglomerating. In addition, CS NPs coated on SiO2 provide an abundance of functional groups (amino and hydroxyl groups) for heavy metal ions [54].
6.3. Mechanism
Based on the solute affinity, adsorption can occur in three types: physical (physisorption), ion exchange and chemical adsorption (chemisorption). Physical adsorption is the result of mainly Van der Waals attraction due to the electrostatic forces between the adsorbate and the surface of the adsorbent. Ion exchange adsorption depends on electrostatic forces occurring between ions retained on the surface, while chemisorption is the result of covalent bonds occurring via chemical interaction between adsorbate and adsorbent. Chemisorption is highly selective, i.e., it occurs between specific adsorptive and adsorbent species, particularly when the active sites are not blocked by previously adsorbed molecules [45].
CS and its nanocomposites can remove heavy metal ions through various mechanisms, such as electrostatic interaction, ionic exchange, metal chelation, and ion-pair formation [55][56]. The adsorption of CS/Fe3O4 NPs is mainly due to the ion exchange on the hydroxyl groups of CS/Fe3O4 NPs and the chelation action of the abundant amine groups in CS [57]. CS is a natural bio-sorbent for toxic metallic ions due to a large number of hydroxyl and amino groups that serve as sorption sites and the flexibility of the polymer chain structure that enables complexation with metal ions [58].
This entry is adapted from the peer-reviewed paper 10.3390/polym15061453
References
- Ma, X.; Zhao, S.; Tian, Z.; Duan, G.; Pan, H.; Yue, Y.; Li, S.; Jian, S.; Yang, W.; Liu, K.; et al. MOFs meet wood: Reusable magnetic hydrophilic composites toward efficient water treatment with super-high dye adsorption capacity at high dye concentration. Chem. Eng. J. 2022, 446, 136851.
- Mahmoodi, N.M.; Arami, M.; Bahrami, H.; Khorramfar, S. Novel biosorbent (Canola hull): Surface characterization and dye removal ability at different cationic dye concentrations. Desalination 2010, 264, 134–142.
- Mahmoodi, N.M.; Arami, M. Modeling and sensitivity analysis of dyes adsorption onto natural adsorbent from colored textile wastewater. J. Appl. Polym. Sci. 2008, 109, 4043–4048.
- Schweitzer, L.; Noblet, J. Chapter 3.6—Water Contamination and Pollution. In Green Chemistry; Török, B., Dransfield, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 261–290.
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067.
- Yu, H.; Wu, M.; Duan, G.; Gong, X. One-step fabrication of eco-friendly superhydrophobic fabrics for high-efficiency oil/water separation and oil spill cleanup. Nanoscale 2022, 14, 1296–1309.
- Zheng, Q.; Li, Z.; Watanabe, M. Production of solid fuels by hydrothermal treatment of wastes of biomass, plastic, and biomass/plastic mixtures: A review. J. Bioresour. Bioprod. 2022, 7, 221–244.
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Singh Sidhu, G.P.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y.; et al. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364.
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610.
- Wan Ngah, W.S.; Hanafiah, M.A.K.M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol. 2008, 99, 3935–3948.
- Sun, Y.; Liu, R.; Wen, S.; Wang, J.; Chen, L.; Yan, B.; Peng, S.; Ma, C.; Cao, X.; Ma, C.; et al. Antibiofouling Ultrathin Poly(amidoxime) Membrane for Enhanced U(VI) Recovery from Wastewater and Seawater. ACS Appl. Mater. Interfaces 2021, 13, 21272–21285.
- Begum, S.; Yuhana, N.Y.; Md Saleh, N.; Kamarudin, N.H.N.; Sulong, A.B. Review of chitosan composite as a heavy metal adsorbent: Material preparation and properties. Carbohydr. Polym. 2021, 259, 117613.
- Malayoglu, U. Removal of heavy metals by biopolymer (chitosan)/nanoclay composites. Sep. Sci. Technol. 2018, 53, 2741–2749.
- Pooja, G.; Kumar, P.S.; Indraganti, S. Recent advancements in the removal/recovery of toxic metals from aquatic system using flotation techniques. Chemosphere 2022, 287, 132231.
- Gao, J.; Yuan, Y.; Yu, Q.; Yan, B.; Qian, Y.; Wen, J.; Ma, C.; Jiang, S.; Wang, X.; Wang, N. Bio-inspired antibacterial cellulose paper–poly(amidoxime) composite hydrogel for highly efficient uranium(VI) capture from seawater. Chem. Commun. 2020, 56, 3935–3938.
- Trung, T.S.; Tram, L.H.; Van Tan, N.; Van Hoa, N.; Minh, N.C.; Loc, P.T.; Stevens, W.F. Improved method for production of chitin and chitosan from shrimp shells. Carbohydr. Res. 2020, 489, 107913.
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393.
- Tokatlı, K.; Demirdöven, A. Optimization of chitin and chitosan production from shrimp wastes and characterization. J. Food Process. Preserv. 2018, 42, e13494.
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98.
- Singh Dhillon, G.; Kaur, S.; Jyoti Sarma, S.; Kaur Brar, S.; Verma, M.; Yadagiri Surampalli, R. Recent Development in Applications of Important Biopolymer Chitosan in Biomedicine, Pharmaceuticals and Personal Care Products. Curr. Tissue Eng. 2013, 2, 20–40.
- Yang, H.; Yan, N. Transformation of Seafood Wastes into Chemicals and Materials. In Green Chemistry and Chemical Engineering; Han, B., Wu, T., Eds.; Springer: New York, NY, USA, 2019; pp. 461–482.
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, Chemical Modification and Characterization of Chitin and Chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189.
- El Knidri, H.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and efficient extraction of chitin and chitosan for scale-up production: Effect of process parameters on deacetylation degree and molecular weight. Int. J. Biol. Macromol. 2019, 139, 1092–1102.
- Stephen, A.M.; Phillips, G.O. Food Polysaccharides and Their Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016.
- No, H.K.; Meyers, S.P. Preparation and characterization of chitin and chitosan—A review. J. Aquat. Food Prod. Technol. 1995, 4, 27–52.
- Fernández-Saiz, P.; Lagaron, J.M. Chitosan for Film and Coating Applications. In Biopolymers—New Materials for Sustainable Films and Coatings; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 87–105.
- Li, D.; Tian, X.; Wang, Z.; Guan, Z.; Li, X.; Qiao, H.; Ke, H.; Luo, L.; Wei, Q. Multifunctional adsorbent based on metal-organic framework modified bacterial cellulose/chitosan composite aerogel for high efficient removal of heavy metal ion and organic pollutant. Chem. Eng. J. 2020, 383, 123127.
- Chen, C.S.; Liau, W.Y.; Tsai, G.J. Antibacterial effects of N-sulfonated and N-sulfobenzoyl chitosan and application to oyster preservation. J. Food Prot. 1998, 61, 1124–1128.
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795.
- Yeul, V.S.; Rayalu, S.S. Unprecedented Chitin and Chitosan: A Chemical Overview. J. Polym. Environ. 2013, 21, 606–614.
- Sikorski, D.; Gzyra-Jagieła, K.; Draczyński, Z. The Kinetics of Chitosan Degradation in Organic Acid Solutions. Mar. Drugs 2021, 19, 236.
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J. 2021, 159, 110709.
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632.
- Lv, S.H. 7—High-performance superplasticizer based on chitosan. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Pacheco-Torgal, F., Ivanov, V., Karak, N., Jonkers, H., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 131–150.
- Winterowd, J.G.; Sandford, P.A. Food Polysaccharides and Their Applications; Stephen, A.M., Ed.; Marcel Dekker: New York, NY, USA, 1995.
- Tharanathan, R.N.; Kittur, F.S. Chitin—The Undisputed Biomolecule of Great Potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87.
- Li, B.; Elango, J.; Wu, W. Recent Advancement of Molecular Structure and Biomaterial Function of Chitosan from Marine Organisms for Pharmaceutical and Nutraceutical Application. Appl. Sci. 2020, 10, 4719.
- El-hefian, E.A.; Nasef, M.M.; Yahaya, A.H. Chitosan physical forms: A short review. Aust. J. Basic Appl. Sci. 2011, 5, 670–677.
- Shaumbwa, V.R.; Liu, D.; Archer, B.; Li, J.; Su, F. Preparation and application of magnetic chitosan in environmental remediation and other fields: A review. J. Appl. Polym. Sci. 2021, 138, 51241.
- Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M.; Anitha, K.L. Recent advances in heavy metal removal by chitosan based adsorbents. Carbohydr. Polym. 2021, 251, 117000.
- Le, T.T.N.; Le, V.T.; Dao, M.U.; Nguyen, Q.V.; Vu, T.T.; Nguyen, M.H.; Tran, D.L.; Le, H.S. Preparation of magnetic graphene oxide/chitosan composite beads for effective removal of heavy metals and dyes from aqueous solutions. Chem. Eng. Commun. 2019, 206, 1337–1352.
- Saleh, T.A.; Mustaqeem, M.; Khaled, M. Water treatment technologies in removing heavy metal ions from wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100617.
- Song, X.; Li, L.; Zhou, L.; Chen, P. Magnetic thiolated/quaternized-chitosan composites design and application for various heavy metal ions removal, including cation and anion. Chem. Eng. Res. Des. 2018, 136, 581–592.
- Anderson, A.; Anbarasu, A.; Pasupuleti, R.R.; Manigandan, S.; Praveenkumar, T.R.; Aravind Kumar, J. Treatment of heavy metals containing wastewater using biodegradable adsorbents: A review of mechanism and future trends. Chemosphere 2022, 295, 133724.
- Samoila, P.; Humelnicu, A.C.; Ignat, M.; Cojocaru, C.; Harabagiu, V. Chitin and Chitosan for Water Purification. In Chitin and Chitosan; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 429–460.
- Feng, M.; Zhang, P.; Zhou, H.-C.; Sharma, V.K. Water-stable metal-organic frameworks for aqueous removal of heavy metals and radionuclides: A review. Chemosphere 2018, 209, 783–800.
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10.
- Ray, P.Z.; Shipley, H.J. Inorganic nano-adsorbents for the removal of heavy metals and arsenic: A review. RSC Adv. 2015, 5, 29885–29907.
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137.
- Charpentier, T.V.J.; Neville, A.; Lanigan, J.L.; Barker, R.; Smith, M.J.; Richardson, T. Preparation of Magnetic Carboxymethylchitosan Nanoparticles for Adsorption of Heavy Metal Ions. ACS Omega 2016, 1, 77–83.
- Jiang, Z.; Li, N.; Li, P.-Y.; Liu, B.; Lai, H.-J.; Jin, T. One-Step Preparation of Chitosan-Based Magnetic Adsorbent and Its Application to the Adsorption of Inorganic Arsenic in Water. Molecules 2021, 26, 1785.
- Karimi, F.; Ayati, A.; Tanhaei, B.; Sanati, A.L.; Afshar, S.; Kardan, A.; Dabirifar, Z.; Karaman, C. Removal of metal ions using a new magnetic chitosan nano-bio-adsorbent; A powerful approach in water treatment. Environ. Res. 2022, 203, 111753.
- Zhang, H.; Li, Y.; Shi, R.; Chen, L.; Fan, M. A robust salt-tolerant superoleophobic chitosan/nanofibrillated cellulose aerogel for highly efficient oil/water separation. Carbohydr. Polym. 2018, 200, 611–615.
- Liu, J.; Chen, Y.; Han, T.; Cheng, M.; Zhang, W.; Long, J.; Fu, X. A biomimetic SiO2@chitosan composite as highly-efficient adsorbent for removing heavy metal ions in drinking water. Chemosphere 2019, 214, 738–742.
- Aizat, M.A.; Aziz, F. 12—Chitosan Nanocomposite Application in Wastewater Treatments. In Nanotechnology in Water and Wastewater Treatment; Ahsan, A., Ismail, A.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 243–265.
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Guna, V.K.; Gopalakrishna, K.; Kumar K., Y. Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohydr. Polym. 2016, 153, 600–618.
- Fan, H.L.; Zhou, S.F.; Jiao, W.Z.; Qi, G.S.; Liu, Y.Z. Removal of heavy metal ions by magnetic chitosan nanoparticles prepared continuously via high-gravity reactive precipitation method. Carbohydr. Polym. 2017, 174, 1192–1200.
- Al-Shahrani, H.; Alakhras, F.; Al-Abbad, E.; Al-Mazaideh, G.; Hosseini-Bandegharaei, A.; Ouerfelli, N. Sorption of cobalt (II) ions from aqueous solutions using chemically modified chitosan. Glob. Nest J. 2018, 20, 620–627.
This entry is offline, you can click here to edit this entry!
