Cellulose nanocrystals (CNC), also known as nanocrystalline cellulose (NCC), cellulose nanowhiskers (CNW), or cellulose crystallites, are obtained by acid hydrolysis of wood or various non-wood materials (cotton, hemp, flax, wheat straw, mulberry bark, ramie, Avicel, tunicin, algae or bacteria), which consists in the chemical removal of lignin, hemicellulose and of amorphous regions within cellulose [
12]. Cellulose nanofibrils (CNFs), also called microfibrillated cellulose, nanofibrils, and microfibrils, or nanofibrillated cellulose (NFC), are extracted from wood, sugar beet, potato tuber, hemp, flax by delamination of pulp by mechanical pressure before and/or after chemical or enzymatic treatment [
9].
Bacterial nanocellulose (BNC) or microbial cellulose is produced by some bacterial genera, such as Gram-negative bacteria:
Acetobacter,
Rhizobium,
Pseudomonas,
Salmonella, etc. or Gram-positive bacteria:
Sarcina ventriculi, in fermentation processes of sugars and vegetable carbohydrates. This has a large specific surface area, higher water retention value, is considered chemically pure cellulose, and does not contain lignin or hemicellulose [
13].
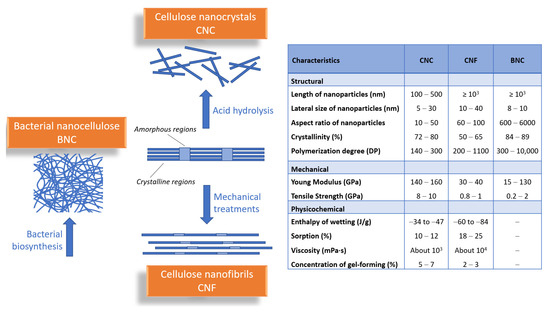
All types of nanocelluloses (NCs) are chemically similar but have different organizational forms and consequently different physical characteristics. As entities, CNFs are micrometer-long fibrils having highly entangled networks of nanofibers with both crystalline and amorphous domains. CNCs are stiffer and highly crystalline rods (ca. 90%), while BNCs are secreted as a ribbon-shaped fibril composed of a bundle of much finer nanofibers.
2. Advanced Functional Materials Based on Nanocellulose—General Characteristics
2.1. Hydrogels
With the progress of nanotechnology, hydrogels have received much more attention due to their particular and excellent characteristics. Hydrogels were the first biomaterials to be conceived for use in humans. They have moved forward to now mimic basic physiological processes and are essentials as bioactive implants in the sense of “in vivo” scaffolds.
The use of hydrogels as biomaterials is strongly related to their properties. Hydrogels are three-dimensional network colloidal gels from hydrophilic polymer crosslinked able by swelling to absorb and retain large volumes of water in an aqueous environment [
18,
19,
20,
21,
22,
23]. In the swollen state, they have a soft and rubbery structure that mimics the behavior of extracellular matrix (ECM) in biological tissues [
24]. Furthermore, hydrogels are conformable to different kinds of surfaces on which they are placed. These properties, in combination with their mucoadhesive nature, elasticity, swelling, and deswelling characteristics in response to environmental stimuli, make hydrogels potential candidates for biomedical applications [
18,
25]. Thus, hydrogels have found applications to produce different types of materials such as contact lenses [
26], blood-contacting hydrogels [
27], wound-healing bioadhesives [
28], artificial kidney membranes [
29], artificial skin [
30], vocal cord replacement [
31,
32], and artificial tendons [
33].
Depending on the size of the obtained particles, hydrogels may be classified as macro-, micro-, or nanogels. When they have particle sizes bigger than 100 m, they are usually called macrogels, while gels with particle sizes up to the micrometer range are called microgels. Finally, if these gels are smaller than 100 nm, they are usually considered nanogels [
34,
35].
2.2. Nanogels
Nanogels, also called “hydrogel nanoparticles”, “nanoscalar polymer networks”, “gel nanoparticles”, or “nanoscale hydrogels”, are combine the properties of gels with those of colloids [
18]. Generally, they have a spherical shape and size between 20 and 200 nm [
36].
Hydrogels, at the nanometer scale, have a great potential in the field of biomedical applications, e.g., as drug-delivery systems, as they combine the characteristics of hydrogels with the advantages of nanoparticles [
36,
37]. Reducing the size of hydrogel particles in the nano range is reflected in increasing the solubility of hydrophobic drugs, improving the accumulation of drugs in tumors, but also by reducing cytotoxic side effects and increasing the stability of therapeutic agents against enzymatic and chemical degradation [
34]. The nanogels also possess some desirable properties, such as high drug-loading capacities, chemical stability, and mechanical properties to avoid the disassembly or fracture during transport, and sensitive response behavior to ensure rapid drug release in response to the relevant stimuli [
38].
In addition to their excellent applicability in the drug delivery field, nanogels have also found applications in other biomedical fields, such as chemotherapy [
39,
40], diagnosis of diseases [
41], vaccines delivery [
42], biocatalysis [
43], and generation of bioactive scaffolds in regenerative medicine [
34]. They have also been studied for use in diabetes treatments [
44] and gene and protein delivery [
41,
45].
Nanohydrogels prepared from natural sources have drawn huge attention due to their vast applications in pharmacy, medicine, tissue engineering, cancer therapy, and drug delivery [
46]. The use of nanocellulosic materials in obtaining hydrogels from renewable materials has been a much-desired goal that has been achieved for many hydrogel types [
47,
48,
49]. Several smart hydrogels such as injectable hydrogels [
33,
50,
51], shape memory [
52,
53], supramolecular hydrogels [
54,
55], double-membrane hydrogels [
56], temperature-sensitive hydrogels [
57], and many other hydrogels types based on nanocellulose with potential for biomedical applications have been developed.
However, on their own, nanocellulose materials do not gel [
58]. Different ways are used to perform, for example, for the gelation of CNC suspensions: by simply increasing the concentration of suspension due to a decrease in the electrostatic double-layer distance [
59]; by modifying the solvent conditions through ionic strength increase [
60]; by addition of polymers [
58]; by sonication [
61]; and by hydrothermal treatment at elevated temperature [
47].
Nanocrystalline cellulose, with their high rigidity and relatively low anisotropy, are well-suited to act as templates for aligned structures (e.g., artificial muscle-like materials) while providing toughness and flexibility. With collagen, for instance, this afforded networks with mechanical properties similar to tendon and ligaments and excellent biocompatibility [
62]. Nanofibrillated cellulose (CNFs) is the type of nanocellulose most likely to form hydrogels due to the length of the nanofibrils. CNFs form gels with much higher elasticity than those resulting from CNCs. CNF suspensions exhibit gelation (G′ > G″, G′ ∝ ω
0, and G″ ∝ ω
0, where, G′ is the storage modulus, G″ is the loss modulus, ω is the frequency) even down to a concentration near to 0.1 wt.%, i.e., the critical gelation concentration, above which the nanofibrils form interconnected networks [
63]. CNFs will afford such structures at concentration ranges of 0.05–6 wt.% [
62]. The simplest case is offered by pristine CNFs, which spontaneously form hydrogels, probably promoted by their length and interacting entanglements [
64].
2.3. Nanocomposites
Currently, research is also progressing in the field of nanocomposite hydrogels, including functionalized nanomaterials [
18]. In general, in order to improve or modify certain properties, polymeric matrices of nanocomposites are reinforced with nanoparticles/nanofillers [
65]. In particular, hydrogels are reinforced with nanoscale materials to obtain nanocomposites with high mechanical strength characteristics or are combined with nanoparticles that confer antibacterial or magnetic properties [
34].
2.3.1. Nanocellulose Materials as “Reinforcing Agents” into Polymer Matrices
Over the past decade, composite materials have attracted a great deal of interest, and particular attention has been focused on the use of nanocellulose as an alternative to inorganic reinforcing agents in polymer matrices for the production of fully “green” composites [
66,
67,
68]. Nanocellulose, owing to its exceptionally high mechanical properties (high specific strength and modulus), high surface area, high aspect ratio, and low environmental impact, has greater advantages as reinforcing filler in comparison to glass fibers, silica, carbon black, and other expensive nanosized fillers [
65]. Thus, composite materials with natural fillers have not only met the environmental appeal but also contributed to developing low-density materials with improved properties [
69].
Hydrogels entirely made of biopolymers and reinforced with nanocellulose can be classified as “green” nanocomposite materials because of their renewable and biodegradable design [
70]. The design of cellulose-based biocomposites is a pathway with many alternatives due to the wide variety of cellulose fibers with specific geometries, the diversity of polymers and manufacturing processes, the multitude of types of reinforcements, and the possibilities of orientation and arrangement of fibers [
17].
Nanocrystalline cellulose has been investigated as reinforcing agents for a variety of polymeric systems due to their large aspect ratio, high specific strength and modulus, low density, high surface area, and unique optical properties [
51,
62,
65,
71,
72]. CNCs have been used as reinforcing agents in a wide range of polymer matrices, from the most common to the most unusual, such as: poly(vinyl alcohol), poly(oxyethylene), polyethylene glycol, poly(
N-isopropylacrylamide), starch, natural rubber, or polyurethane [
67,
73,
74,
75,
76].
Nanofibrillated cellulose (NFC) has excellent properties for mechanical reinforcement due to its special morphology that combines the advantages of the length of the fibers in the micrometers range with those of their width in the nanometers range [
69]. The use of CNF networks as reinforcing elements together with a suitable matrix polymer is an efficient reinforcement solution for high-quality, specialized applications of bio-based composites. The combination of nanofiber flexibility, aspect ratio, and strength is the main advantage of CNFs in various applications [
77]. For instance, comparing the reinforcement capacity of CNC and NFC (at the same addition) using poly(ethylene oxide) (PEO) as the polymer matrix, Xu and coworkers [
75] reported that the nanocomposites reinforced with NFC demonstrated higher-strength and elastic modulus than nanocomposites with CNC. However, CNC-based nanocomposites presented a higher strain of failure. The higher strength values of NFC-based nanocomposites are the effect of their high aspect ratio of cellulose nanofibrils that favors more entanglement and network percolation.
By comparison, bacterial nanocellulose (BNC), having the highest purity of all nanocellulose materials, doubled by high crystallinity and excellent biological affinity, is the ideal reinforcing component for biopolymer composites [
78].
2.3.2. Nanocellulose Materials as “Matrices” for Different Reinforcing Agents
The inclusion of biocompatible and/or bioactive compounds as components of the composite is the proper way to overcome certain limitations of nanocellulose materials, improving their biocompatibility, antimicrobial activity, or water-holding capacity [
79].
Generally, nanocellulose can be reinforced with different polymers with specific properties, obtaining a material with different characteristics from those of starting materials [
65]. Nanocellulose can also be used as a substrate for the incorporation of inorganic nanoparticles, such as carbon nanotubes, graphene, and graphene oxide to obtain hydrogels with antibacterial, antiviral, antifungal, magnetic, electrical, and mechanical properties. The high specific surface area, the presence of reducing functional groups, and the ability to form aqueous suspensions are the main arguments for the use of nanocelluloses as a support for metal/metal oxide nanoparticles [
80]. The process of composite formation is performed through physicochemical interactions or by mechanical capture of nanoparticles in the structural matrix of nanocellulose [
81].
Nanocellulose-based compounds have found applications in various biomedical areas, from dressings to drug administration and even as a basis for scaffolding in regenerative medicine [
82]. Silver particles have been used as potential agents with a broad antibacterial activity and low presumed toxicity to coat cellulosic materials for biomedical applications. The composite was prepared by immersing BNC in a silver ammonium solution and showed to be effective as dressing in wound-healing applications by decreasing inflammation and promoting wound-healing [
30]. The silver nanoparticles were incorporated in crystalline nanocellulose by microwave-assisted synthesis, and the composites proved to have high antibacterial properties against
E. coli (Gram-negative bacteria) and
S. aureus (Gram-positive bacteria) [
83]. Barua and coworkers [
84] prepare copper-copper oxide nanoparticles (Cu–CuO) NP-coated CNFs through a green reductive technique, which exhibited promising antimicrobial activity against Gram-positive and Gram-negative bacteria and fungal species.
3. Nanocellulose-Based Materials in Pharmaceutical/Medical Applications
The nanocellulose materials, used as independent functional material or as reinforcement units in composite materials, have received tremendous attention in a wide variety of applications, including foods, packaging, cosmetics, biomedical implants, optics, water filtration, hygienic applications, and so forth [
3,
14,
17]. However, especially in biomedical fields, they appear to have significant advantages due to their intrinsic biodegradability and biocompatibility [
14]. However, other interesting features should also be considered, such as mechanical properties, low risk of cytotoxicity, its three-dimensional (3D) nanofibrous network, and last but not least, its natural source [
62,
85,
86].
3.1. Nanocellulose-Based Materials in Drug-Delivery Systems (DDS)
An ideal drug carrier should be nontoxic, non-immunogenic, biocompatible, and biodegradable; enhance drug solubility and stability and have high drug-loading capacity; and be capable of reaching correct concentrations at a proper rate determined by an optimal [
36]. Other equally important criteria to be met are related to its size and surface characteristics because these two parameters control the residence time in the bloodstream and the target site. More exactly, the size needs to be sufficiently large enough to prevent rapid penetration into fenestrated blood vessels, yet sufficiently small to avoid phagocytosis. The surface nature also decides the duration and destination of the drug carrier in the circulatory system. For instance, a hydrophilic surface will most likely make the carrier avoid phagocytosis by macrophages, and this hydrophilicity can be accomplished either by covering the surface with a hydrophilic polymer (i.e., PEG) or by using block copolymers with hydrophilic and hydrophobic areas [
62].
Being a natural nanosized material, nanocellulose features meet the necessary criteria mentioned above regarding its function as a vehicle for DDS: its horizontal measurements extend from 5 to 20 nm, and the longitudinal measurement ranges from 10 nm to a few microns; each of its monomers bears three hydroxyl groups with the ability to form hydrogen bonds, which plays a major role in the surface hydrophilicity. Of course, the nanocellulose unique properties should not be overlooked because they make this material play an important role among drug-delivery vectors, such as high crystallinity, biocompatibility, biodegradability, high surface area, unique mechanical and rheological properties, liquid absorption capacity, and porosity [
36,
85,
87,
88].
The three types of nanocellulose are quite similar to each other, yet there are distinct differences that set them apart. These differences make each type of nanocellulose better suited for a certain drug-delivery system compared to the others [
87]. We summarize the recent reports on nanocellulose-based drug-delivery systems in
Table 1.
Table 1. Nanocellulose hydrogels/nanocomposites in drug-delivery applications.