Over the past century and a half, the taxonomic placement of Eriophyoidea has been in flux. For much of this period, this group has been treated as a subtaxon within Trombidiformes. However, the vast majority of phylogenetic analyses, including almost all phylogenomic analyses, place this group outside Trombidiformes. The few studies that still place Eriophyoidea within Trombidiformes are likely to be biased by incomplete taxon/gene sampling, long branch attraction, the omission of RNA secondary structure in sequence alignment, and the inclusion of hypervariable expansion–contraction rRNA regions. Based on the agreement among a number of independent analyses that use a range of different datasets (morphology; multiple genes; mitochondrial/whole genomes), Eriophyoidea are almost certain to be closely related to Nematalycidae, a family of vermiform mites within Endeostigmata, a basal acariform grade. Much of the morphological evidence in support of this relationship was apparent after the discovery of Nematalycidae in the middle of the 20th century.
- Acariformes
- Eriophyoidea
- Nematalycidae
- Phylogenomics
- Trombidiformes
1. Introduction
2. The Morphological Era (1877–2015)
3. Molecular Era (2016–Present)
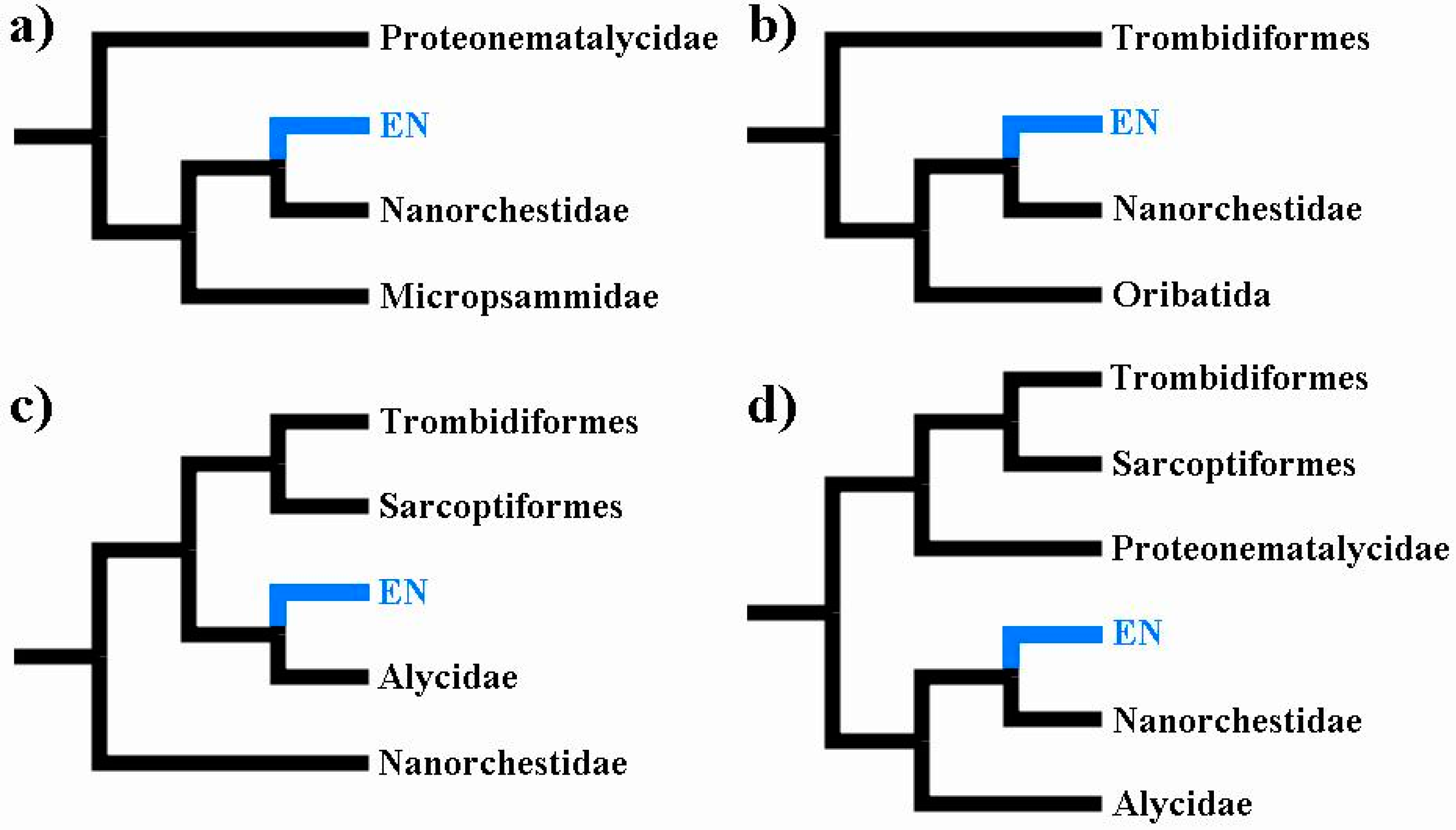
4. Are Eriophyoidea Nested within Tydeoidea?
This entry is adapted from the peer-reviewed paper 10.3390/insects14060527
References
- Zhang, Z.-Q. Eriophyoidea and Allies: Where Do They Belong? Syst. Appl. Acarol. 2017, 22, 1091–1095.
- Xue, X.-F.; Guo, J.-F.; Dong, Y.; Hong, X.-Y.; Shao, R. Mitochondrial Genome Evolution and tRNA Truncation in Acariformes Mites: New Evidence from Eriophyoid Mites. Sci. Rep. 2016, 6, 18920.
- Klimov, P.B.; Chetverikov, P.E.; Dodueva, I.E.; Vishnyakov, A.E.; Bolton, S.J.; Paponova, S.S.; Lutova, L.A.; Tolstikov, A.V. Symbiotic Bacteria of the Gall-Inducing Mite Fragariocoptes Setiger (Eriophyoidea) and Phylogenomic Resolution of the Eriophyoid Position among Acari. Sci. Rep. 2022, 12, 3811.
- Pepato, A.R.; Costa, S.G.D.S.; Harvey, M.S.; Klimov, P.B. One-Way Ticket to the Blue: A Large-Scale, Dated Phylogeny Revealed Asymmetric Land-to-Water Transitions in Acariform Mites (Acari: Acariformes). Mol. Phylogenet. Evol. 2022, 177, 107626.
- Klimov, P.B.; OConnor, B.M.; Chetverikov, P.E.; Bolton, S.J.; Pepato, A.R.; Mortazavi, A.L.; Tolstikov, A.V.; Bauchan, G.R.; Ochoa, R. Comprehensive Phylogeny of Acariform Mites (Acariformes) Provides Insights on the Origin of the Four-Legged Mites (Eriophyoidea), a Long Branch. Mol. Phylogenet. Evol. 2018, 119, 105–117.
- Arribas, P.; Andújar, C.; Moraza, M.L.; Linard, B.; Emerson, B.C.; Vogler, A.P. Mitochondrial Metagenomics Reveals the Ancient Origin and Phylodiversity of Soil Mites and Provides a Phylogeny of the Acari. Mol. Biol. Evol. 2020, 37, 683–694.
- Li, W.-N.; Xue, X.-F. Mitochondrial Genome Reorganization Provides Insights into the Relationship between Oribatid Mites and Astigmatid Mites (Acari: Sarcoptiformes: Oribatida). Zool. J. Linn. Soc. 2019, 187, 585–598.
- Xue, X.-F.; Dong, Y.; Deng, W.; Hong, X.-Y.; Shao, R. The Phylogenetic Position of Eriophyoid Mites (superfamily Eriophyoidea) in Acariformes Inferred from the Sequences of Mitochondrial Genomes and Nuclear Small Subunit (18S) rRNA Gene. Mol. Phylogenet. Evol. 2017, 109, 271–282.
- Fang, Y.; Fang, Y.; Chu, L.; Zuo, Z.; Liu, L.; Feng, R.; Zhang, Z.; Zhan, X.; Li, F.; Hu, C.; et al. The First Complete Mitochondrial Genome of Bdelloidea (Trombidiformes, Eupodina) and Comparative Genomics Provide Insights into Gene Rearrangement and Evolution of Trombidiform Mites. J. Stored Prod. Res. 2022, 98, 102009.
- Greenhalgh, R.; Dermauw, W.; Glas, J.J.; Rombauts, S.; Wybouw, N.; Thomas, J.; Alba, J.M.; Pritham, E.J.; Legarrea, S.; Feyereisen, R.; et al. Genome Streamlining in a Minute Herbivore That Manipulates Its Host Plant. eLife 2020, 9, e56689.
- Bolton, S.J.; Chetverikov, P.E.; Klompen, H. Morphological Support for a Clade Comprising Two Vermiform Mite Lineages: Eriophyoidea (Acariformes) and Nematalycidae (Acariformes). Syst. Appl. Acarol. 2017, 22, 1096–1131.
- Murray, A. Economic Entomology: Aptera; Chapman & Hall: London, UK, 1877.
- Kramer, P. Grundzüge Zur Systematik Der Milben. Arch. Naturgesch. 1877, 43, 215–247.
- Claus, C.; Moquin-Tandon, G. Traité De Zoologie, Traduction Française, 2nd ed.; Librairie F. Savy: Paris, France, 1884.
- Canestrini, G. Abbozzo del Sistema Acarologico. In Atti del Reale Istituto Veneto di Scienzem Lettere ed Arti; University of Bern: Bern, Switzerland, 1891; Volume 7, pp. 699–725.
- Oudemans, A.C. Nieuwe Classificatie Der Acari. Entomolog. Ber. 1906, 2, 43–46.
- Reuter, E. Zur Morphology Und Ontogenie Der Acariden Mit Besonderer Berücksichtigung von Pendiculopsis Graminum. Acta Soc. Scient. Fenn. 1909, 36, 1–288.
- Evans, G.O. Principles of Acarology; CAB International: Wallingford, UK, 1992.
- Baker, E.W.; Wharton, G.W. An Introduction to Acarology; The Macmillan Co.: New. York, NY, USA, 1952; 465p.
- Baker, E.W. A New Trichadenid Mite Which Further Indicates a Phylogenetic Relationship between the Tetranychidae and Eriophyidae. Proc. Entomol. Soc. Wash. 1948, 50, 59–60.
- Keifer, H.H. The Eriophyoidea Nalepa. In Mites Injurious Eriophyoid Mites; Jeppson, L.R., Keifer, H.H., Baker, E.W., Eds.; University of California Press: Berkley, CA, USA, 1975; pp. 327–587.
- Strenzke, K. Nematalycus Nematoides N. Gen. N. Sp. (Acarina Trombidiformes) Aus Dem Grundwasser Der Algerischen Kuste. Vie Milieu Paris 1953, 4, 638–647.
- Cunliffe, F. A New Species of Nematalycus Strenzke with Notes on the Family (Acarina, Nematalycidae). Proc. Entomol. Soc. Wash. 1956, 58, 353–355.
- Wainstein, B.A. The System of the Aquatic Mites and Their Place in the Suborder Trombidiformes. Tr. Inst. Biol. Vnutr. Vod 1965, 8, 66–83.
- Krantz, G.W. A Manual of Acarology; Oregon State University Book Stores: Corvallis, OR, USA, 1971; 335p.
- Kethley, J. Acariformes-Prostigmata. In Synopsis and Classification of Living Organisms; Stony Brook University: Stony Brook, NY, USA, 1982; Volume 2, pp. 117–145.
- Kethley, J.B. Acarina: Prostigmata (Actinedida). In Soil Biology Guide; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1990; pp. 667–756.
- Bolton, S.J.; Bauchan, G.R.; Ochoa, R.; Klompen, H. A Novel Fluid-Feeding Mechanism for Microbivory in the Acariformes (Arachnida: Acari). Arthropod Struct. Dev. 2015, 44, 313–325.
- Bolton, S.J.; Bauchan, G.R.; Chetverikov, P.E.; Ochoa, R.; Klompen, H. A Rudimentary Sheath for the Smallest of “biting” Chelicerae: The Mouthparts of Cunliffea (Nematalycidae) and a New Hypothesis on the Origin of the Stylet Sheath of Eriophyoidea (Acariformes). Int. J. Acarol. 2018, 44, 374–381.
- Lindquist, E.E.; Krantz, G.W.; Walter, D.E. Classification. In A Manual of Acarology: Third Edition; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 97–103.
- Lindquist, E.E. Evolution of Phytophagy in Trombidiform Mites. Exp. Appl. Acarol. 1998, 22, 81–100.
- Kethley, J. Proteonematalycidae (Acari: Acariformes), a New Mite Family from Fore-Dune Sand of Lake Michigan. Int. J. Acarol. 1989, 15, 209–217.
- Lindquist, E.E. 1.5.2 Phylogenetic Relationships. In World Crop Pests; Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 6, pp. 301–327. ISBN 9780444886286.
- Nuzzaci, G.; de Lillo, E. Linee Evolutive Dello Gnatosoma in Alcuni Acari Prostigmata. In Atti XVI Congresso Nazionale Italiano di Entomologia; Scientific Press: Christchurch, New Zealand, 1991.
- Zhang, Z.; Fan, Q.H.; Pesic, V.; Smit, H. Order Trombidiformes Reuter, 1909. In Zhang, Z.-Q. (Ed.) Animal Biodiversity: An Outline of Higher-Level Classification and Survey of Taxonomic Richness. Zootaxa 2011, 3148, 129.
- Thia, J.A.; Young, N.D.; Korhnen, P.K.; Yang, Q.; Gasser, R.B.; Umina, P.A.; Hoffmann, A.A. The Mitogenome of Halotydeus Destructor (Tucker) and Its Relationships with Other Trombidiform Mites as Inferred from Nucleotide Sequences and Gene Arrangements. Ecol. Evol. 2021, 11, 14162–14174.
- Szudarek-Trepto, N.; Kaźmierski, A.; Skoracka, A.; Lewandowski, M.; Dabert, J. Molecular Phylogeny Supports the Monophyly of the Mite Supercohort Eupodides (Acariformes: Trombidiformes) and Greatly Coincides with Traditional Morphological Definition of the Taxon. Annal. Zool. 2022, 72, 757–786.
- Norton, R.A.; Kethley, J.B.; Johnston, D.E.; OConnor, B.M. Phylogenetic Perspectives on Genetic Systems and Reproductive Modes of Mites. In Evolution and Diversity of Sex Ratio in Insects and Mites; Wrensch, D.L., Ebbert, M.A., Eds.; Chapman & Hall: New York, NY, USA, 1993; pp. 8–99.
- Gillespie, J.J.; Johnston, J.S.; Cannone, J.J.; Gutell, R.R. Characteristics of the Nuclear (18S, 5.8S, 28S and 5S) and Mitochondrial (12S and 16S) rRNA Genes of Apis Mellifera (Insecta: Hymenoptera): Structure, Organization, and Retrotransposable Elements. Insect Mol. Biol. 2006, 15, 657–686.
- Gillespie, J.J.; Yoder, M.J.; Wharton, R.A. Predicted Secondary Structure for 28S and 18S rRNA from Ichneumonoidea (Insecta: Hymenoptera: Apocrita): Impact on Sequence Alignment and Phylogeny Estimation. J. Mol. Evol. 2005, 61, 114–137.
- Pepato, A.R.; Klimov, P.B. Origin and Higher-Level Diversification of Acariform Mites—Evidence from Nuclear Ribosomal Genes, Extensive Taxon Sampling, and Secondary Structure Alignment. BMC Evol. Biol. 2015, 15, 178.
- Chetverikov, P.E.; Craemer, C.; Cvrković, T.; Klimov, P.B.; Petanović, R.U.; Romanovich, A.E.; Sukhareva, S.I.; Zukoff, S.N.; Bolton, S.; Amrine, J. Molecular Phylogeny of the Phytoparasitic Mite Family Phytoptidae (Acariformes: Eriophyoidea) Identified the Female Genitalic Anatomy as a Major Macroevolutionary Factor and Revealed Multiple Origins of Gall Induction. Exp. Appl. Acarol. 2021, 83, 31–68.
- Chetverikov, P.E.; Petanović, R.U. Description of a New Early-Derivative Mite, Pentasetacus Plicatus N. Sp. (Acariformes, Eriophyoidea), and Remarks on the Systematic Position of Pentasetacines. Zootaxa 2016, 4144, 211–226.
- Chetverikov, P.E.; Craemer, C. Gnathosomal Interlocking Apparatus and Remarks on Functional Morphology of Frontal Lobes of Eriophyoid Mites (Acariformes, Eriophyoidea). Exp. Appl. Acarol. 2015, 66, 187–202.
- Nuzzaci, G.; Di Palma, A. Mouthparts of a Tydeid Mite: An Ultrastructural and Functional Investigation. Entomologica 2002, 36, 71–91.
