Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Gastroenterology & Hepatology
Drugs are widely used to treat different diseases in modern medicine, but they are often associated with adverse events. Those located in the gastrointestinal tract are common and often mild, but they can be serious or life-threatening and determine the continuation of treatment. The stomach is often affected not only by drugs taken orally but also by those administered parenterally. The first description of the endoscopic picture of the damage to the gastric mucosa associated with the use of aspirin was published by A. Douthwait and J. Lintoff in 1938.
- gastritis
- drug-induced gastric damage
- NSAIDs
- aspirin
1. Epidemiology
NSAIDs are one of the most commonly prescribed classes of medication with a wide range of indications and availability in over-the-counter forms. According to some studies, the prevalence of NSAID and aspirin use among older people is 24.7% [1]. Gastric erosions occur in approximately half of patients receiving NSAIDs, and peptic ulcer disease occurs in 15–30% of cases. Symptomatic peptic ulcers can be observed in 3–4.5% of patients taking NSAIDs, and serious complications (perforation, obstruction or bleeding) occur in approximately 1.5% of patients after 1 year of treatment [2].
According to two large cohort studies, ESTHER (N = 7737) and British Biobank (N = 213,598), taking low doses of aspirin is an independent risk factor for the development of gastric and duodenal ulcers in the early period after the start of treatment [3]. The stomach and duodenal ulcer risk ratios were found to be 1.82 [1.58–2.11] and 1.66 [1.36–2.04] in the case of British Biobank and 2.83 [1.40–5.71] and 3.89 [1.46–10.42] in the ESTHER study, respectively.
According to data from Spain, the mortality rate associated with the use of NSAIDs or aspirin is 5.6%, which is equivalent to 15.3 cases of death per 100,000 users [4].
2. Risk Factors
Risk factors for the development of NSAID/aspirin-associated gastropathy include >60-year-olds (and, in particular, >70-year-olds), high-dose NSAID treatment, a previous history of peptic ulcers with or without complications, co-therapy with low-dose aspirin, anticoagulants, serotonin re-uptake inhibitors or steroids and H. pylori infection [5][6][7].
3. Mechanism of Gastric Damage
Gastrointestinal-associated NSAID/aspirin damage is based on the blockade of the enzyme cyclooxygenase (COX), which regulates the synthesis of prostaglandins from arachidonic acid. COX exists in two isoforms: structural COX-1 and induced COX-2. The COX-2 isoform is not detected in normal tissues. Its expression is induced by inflammatory mediators (lipopolysaccharides, interleukin-1, tumor necrosis factor alpha, macrophages, monocytes) and causes all the clinical manifestations of inflammatory processes: soreness, fever, swelling and dysfunction. Therefore, it is the blockade of COX-2 that causes the main targeted pharmacological effects of NSAIDs/aspirin, including the anti-inflammatory, analgesic and antipyretic. At the same time, COX-1 blockade induces a systemic decrease in the synthesis of prostaglandins (PGs), which have cytoprotective effects.
It has been established that PGE2 inhibits the formation of H+ ions and pepsinogen in the stomach, reducing the volume of gastric secretion and its acid and peptic activity; however, the main effect is the increase in the production of mucus and bicarbonates, stimulation of the processes of cell proliferation and physiological regeneration of the epitheliocytes of the gastric mucosa [8]. Thus, a decrease in PG synthesis is associated with a decrease in the resistance of the gastric mucosa [9], as well as a reduction in the gastric mucosal blood flow due to the ability of NSAIDs/aspirin to inhibit the synthesis of nitric oxide (NO) through the suppression of the activity of the NO synthetase enzyme [10]. At the same time, a decrease in the formation of PG leads to the activation of the lipoxygenase pathway, with an increase in the synthesis of leukotrienes (LTs), primarily LT-B4, and pro-inflammatory cytokines (C5-compliment, tumor necrosis factor-α), which aggravate the inflammation and ischemia of the gastric mucosa [11][12].
The direct (topical) interaction between NSAIDs and phospholipids and the uncoupling of oxidative phosphorylation in mitochondria cause cell membrane damage, with a disruption of the phospholipid layer and tight junctions. This action increases transcellular permeability. The inhibition of COX, as a systemic effect, reduces microvascular blood flow, and luminal aggressive factors modify and amplify this reaction, leading to inflammation, erosions and ulcers [7].
Depending on their blockade of one COX isoform or another, NSAIDs are divided into those that are selective (inhibiting only COX-2) and non-selective (inhibiting both COX-1 and COX-2). Selective drugs, called “coxibs”, have a less damaging effect on the gastric and duodenal mucosa and were initially used to prevent NSAID-associated damage to the digestive tract. However, it was later discovered that as gastrointestinal risks decrease when taking selective NSAIDs, the risk of fatal cardiovascular events increases [13][14][15]. Considering DIG, it should also be noted that the damaging effects of these drugs can be realized throughout the digestive tract and proceed with an awareness of the involvement of other organs and systems (liver, kidneys, etc.).
The mechanism of development of NSAID-associated gastropathy is shown in Figure 1.
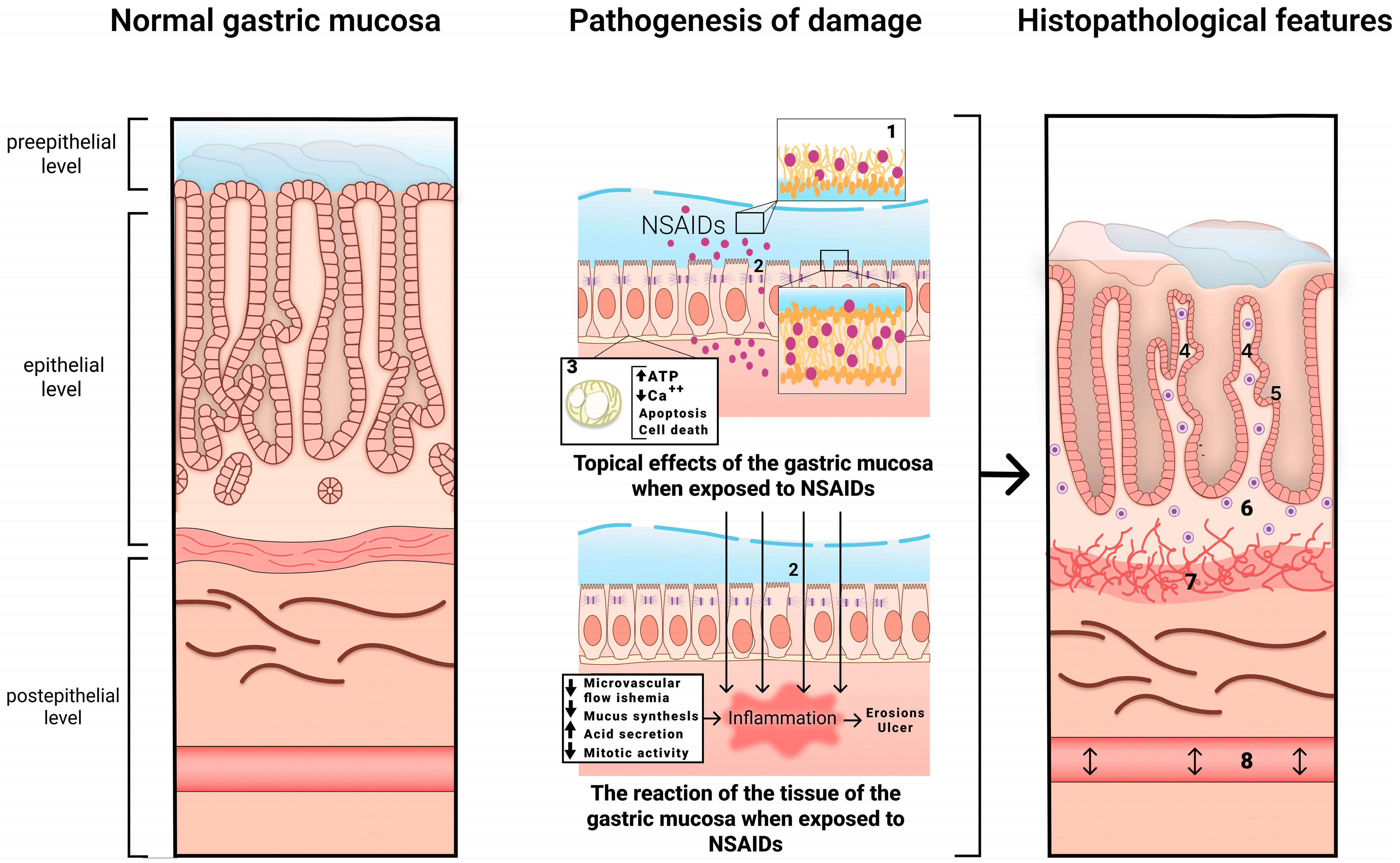
Figure 1. The mechanism of development of NSAID-associated gastropathy. 1—disrupted phospholipid monolayer, 2—damage to tight junction proteins, 3—uncoupled mitochondria, 4—pronounced regenerative changes in the epithelium (foveolar hyperplasia with hyperchromic cell nuclei, decreased mucus formation), 5—subnuclear vacuolated mucous cells, 6—mild diffuse mononuclear infiltration, 7—bundles of smooth muscle cells in the lamina propria, 8—edema with ectatic blood vessels.
4. Clinical Manifestations
As a rule, most patients taking NSAIDs have no gastrointestinal symptoms. However, dyspeptic symptoms may occur in a significant number of patients, including epigastric pain (17–20%) and nausea (22%). In some cases, there might be symptoms such as heartburn, sour belching, constipation (19.3%) or diarrhea (9.2%) showing that other parts of the digestive tract are concerned [16].
A rare but clinically important feature of NSAID/aspirin-associated gastropathy is the development of complications, mainly gastrointestinal bleeding [17][18], with the risk of bleeding being greatest during the first 3 months of taking NSAIDs (OR 11.7; 6.5–21.0) and decreasing with the continued use, becoming minimal 1 week after the deprescribing (OR 3.2; 2.1–5.1) [19][20]. The absolute rate of peptic ulcer bleeding in patients taking these compounds has been reported to be 1% per year, but this rate may be increased substantially in patients with risk factors such as advanced age, a history of peptic ulcers and concomitant use of other drugs, such as anticoagulants, antiplatelet agents, corticosteroids and serotonin re-uptake inhibitors [2][19][20]. Bleeding ulcers can be indicated by the presence of hematemesis and/or melena, but some patients may report only general symptoms of blood loss such as a decrease in blood pressure, tachycardia, pallor of the skin, dizziness or anemia. Some patients with NSAID/aspirin-induced gastropathy may be asymptomatic.
It is important to note that damage to the digestive tract while taking NSAIDs/aspirin is not limited to the mucous membrane of the stomach and duodenum but can also affect the small and large intestines, as has been shown in a number of large, randomized clinical trials [21][22]. Most often, NSAID/aspirin-associated damage to the lower GI tract is accompanied by hidden blood loss and the development of chronic iron deficiency anemia, which aggravates the course of cardiovascular diseases and bronchopulmonary pathology and increases the risk of thromboembolic complications. NSAID/aspirin-associated enteropathy is accompanied, in addition to iron deficiency, by protein loss and hypoalbuminemia. A pathognomonic sign of damage to the small (rarely large) intestine, associated with the long-term use of NSAIDs, is the formation of circular, diaphragm-like strictures as a result of a chronic inflammatory process, which can cause intestinal obstruction [21][23][24].
5. Endoscopic Picture
A typical localization of erosive and ulcerative lesions is the antrum of the stomach, but all areas of the gastroduodenal tract can be affected. This condition is characterized by damage of a multifarious nature, which can be both acute and chronic. Signs of bleeding and subepithelial hemorrhages are often noted [1]. During the healing of an ulcer defect, as a rule, rough scars and deformities do not form [25].
6. Histological Examination
Microscopic signs comprise the picture of the so-called reactive gastropathy, which is not strictly specific to NSAIDs. There is a weak–diffuse, predominantly mononuclear inflammatory infiltration of the lamina propria, often revealing erosive and/or ulcerative defects, pronounced regenerative changes in the epithelium (foveolar hyperplasia with hyperchromic cell nuclei, decreased mucus formation), mucosal edema with vascular ectasia in the lamina propria and lamina propria expansion with fibromuscular proliferation. Subnuclear vacuolated mucous cells may be an additional criterion, which is associated with operated stomach syndrome. Interestingly, the nature of the necrotic masses at the bottom of the defect may be the starting point for the differential diagnosis between NSAID-associated lesions with a homogeneous eosinophilic zone of necrotic masses and a defect caused by Helicobacter pylori (H. pylori) [26] with the presence of necrotic masses that are loosely associated with the lamina propria, with immured fragments, necrotic cells and neutrophilic leukocytes (Figure 2). NSAID exposure, in very rare cases, is accompanied by the formation of diaphragms (diaphragm disease) in the stomach. This phenomenon is more typical of damage to the small and large intestines.

Figure 2. Differential diagnosis between NSAID-associated lesions and a defect caused by Helicobacter pylori. (a) Helicobacter pylori-associated gastric erosion. Inhomogeneous masses of fibrinoid necrosis with cell debris and granulocytes. (b) NSAID-associated gastric erosion. Homogeneous eosinophilic ischemic necrosis blending into the adjacent lamina propria. Hematoxylin and eosin stain ×200.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13132220
References
- Pilotto, A.; Franceschi, M.; Leandro, G.; Di Mario, F.; Geriatric Gastroenterology Study Group (Societe Italiana Gerontologie Geriatria). NSAID and aspirin use by the elderly in general practice: Effect on gastrointestinal symptoms and therapies. Drugs Aging. 2003, 20, 701–710.
- Laine, L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology 2001, 120, 594–606.
- Nguyen, T.N.M.; Sha, S.; Cnen, L.J.; Holleczek, B.; Brenner, H.; Schottker, B. Strongly increased risk of gastric and duodenal ulcers among new users of low-dose aspirin: Results from two large cohorts with new-user design. Aliment. Pharmacol. Ther. 2022, 56, 251–262.
- Lanas, A.; Perez-Aisa, M.A.; Feu, F.; Ponce, J.; Saperas, E.; Santolaria, S.; Rodrigo, L.; Balanzo, J.; Bajador, E.; Almero, P.; et al. A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal anti-inflammatory drug use. Am. J. Gastroenterol. 2005, 100, 1685–1693.
- Joo, M.K.; Park, C.H.; Kim, J.S.; Park, J.M.; Ahn, J.Y.; Lee, B.E.; Lee, J.H.; Yang, H.J.; Cho, Y.K.; Bang, C.S.; et al. Clinical Guidelines for Drug-Related Peptic Ulcer, 2020 Revised Edition. Gut Liver 2020, 14, 707–726.
- Lanas, A. A review of the gastrointestinal safety data-a gastroenterologist’s perspective. Rheumatology 2010, 49, ii3–ii10.
- Bjarnason, I.; Scarpignato, C.; Holmgren, E.; Olszewski, M.; Rainsford, K.D.; Lanas, A. Mechanisms of Damage to the Gastrointestinal Tract FromNonsteroidal Anti-Inflammatory Drugs. Gastroenterology 2018, 154, 500–514.
- Cheng, H.; Huang, H.; Guo, Z.; Chang, Y.; Li, Z. Role of prostaglandin E2 in tissue repair and regeneration. Theranostics 2021, 11, 8836–8854.
- Laporte, J.R.; Ibáñez, L.; Vidal, X.; Vendrell, L.; Leone, R. Upper gastrointestinal bleeding associated with the use of NSAIDs: Newer versus older agents. Drug Saf. 2004, 27, 411–420.
- García Rodríguez, L.A.; Hernández-Díaz, S. Risk of uncomplicated peptic ulcer among users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Am. J. Epidemiol. 2004, 159, 23–31.
- Fiorucci, S.; Antonelli, E.; Morelli, A. Mechanism of non-steroidal anti-inflammatory drug-gastropathy. Dig. Liver Dis. 2001, 33, S35–S43.
- Santucci, L.; Fiorucci, S.; Giansanti, M.; Brunori, P.M.; Di Matteo, F.M.; Morelli, A. Pentoxifylline prevents indomethacin induced acute gastric mucosal damage in rats: Role of tumour necrosis factor alpha. Gut 1994, 35, 909–915.
- Bresalier, R.S.; Sandler, R.S.; Quan, H.; Bolognese, J.A.; Oxenius, B.; Horgan, K.; Lines, C.; Riddell, R.; Morton, D.; Lanas, A.; et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N. Engl. J. Med. 2005, 352, 1092–1102.
- Solomon, S.D.; McMurray, J.J.; Pfeffer, M.A.; Wittes, J.; Fowler, R.; Finn, P.; Anderson, W.F.; Zauber, A.; Hawk, E.; Bertagnolli, M.; et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N. Engl. J. Med. 2005, 352, 1071–1080.
- Coxib and Traditional NSAID Trialists’ (CNT) Collaboration; Bhala, N.; Emberson, J.; Merhi, A.; Abramson, S.; Arber, N.; Baron, J.A.; Bombardier, C.; Cannon, C.; Farkouh, M.E.; et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: Meta-analyses of individual participant data from randomised trials. Lancet 2013, 382, 769–779.
- Maev, I.V.; Andreev, D.N.; Dicheva, D.T.; Partsvania-Vinogradova, E.V. NSAID-induced gastropathies: Pathogenetically substantiated approaches to prevention and therapy. Farmateka 2016, 2, 49–54.
- Laine, L. Proton pump inhibitor co-therapy with nonsteroidal anti-inflammatory drugs--nice or necessary? Rev. Gastroenterol. Disord. 2004, 4, S33–S41.
- Fries, J.F.; Murtagh, K.N.; Bennett, M.; Zatarain, E.; Lingala, B.; Bruce, B. The rise and decline of nonsteroidal antiinflammatory drug-associated gastropathy in rheumatoid arthritis. Arthritis Rheum. 2004, 50, 2433–2440.
- Lanas, Á.; Carrera-Lasfuentes, P.; Arguedas, Y.; García, S.; Bujanda, L.; Calvet, X.; Ponce, J.; Perez-Aísa, Á.; Castro, M.; Muñoz, M.; et al. Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants. Clin. Gastroenterol. Hepatol. 2015, 13, 906–912.e2.
- Lanza, F.L.; Chan, F.K.; Quigley, E.M.; Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am. J. Gastroenterol. 2009, 104, 728–738.
- Otani, K.; Tanigawa, T.; Watanabe, T.; Shimada, S.; Nadatani, Y.; Nagami, Y.; Tanaka, F.; Kamata, N.; Yamagami, H.; Shiba, M.; et al. Microbiota Plays a Key Role in Non-Steroidal Anti-Inflammatory Drug-Induced Small Intestinal Damage. Digestion 2017, 95, 22–28.
- Maiden, L.; Thjodleifsson, B.; Theodors, A.; Gonzalez, J.; Bjarnason, I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology 2005, 128, 1172–1178.
- Zhao, B.; Sanati, S.; Eltorky, M. Diaphragm disease: Complete small bowel obstruction after long-term nonsteroidal anti-inflammatory drugs use. Ann. Diagn. Pathol. 2005, 9, 169–173.
- Grattagliano, I.; Ubaldi, E.; Portincasa, P. Drug-induced enterocolitis: Prevention and management in primary care. J. Dig. Dis. 2018, 19, 127–135.
- Gillen, D.; McColl, K.E. Problems associated with the clinical use of proton pump inhibitors. Pharmacol. Toxicol. 2001, 89, 281–286.
- Stolte, M.; Panayiotou, S.; Schmitz, J. Can NSAID/ASA-induced erosions of the gastric mucosa be identified at histology? Pathol. Res. Pract. 1999, 195, 137–142.
This entry is offline, you can click here to edit this entry!
