Glioblastoma’s poor prognosis calls for the discovery of newer, more efficacious management and treatment methods. Metformin and resveratrol exert anticancer effects on major metabolic pathways in glioblastoma cells, resulting in reduced proliferation, increased apoptosis, and reduced tumor growth and volume. The shown effects suggest that metformin and resveratrol can potentially aid in treating glioblastoma.
1. Metformin and Resveratrol in Glioblastoma
Glioblastoma (GBM) is a malignant brain tumor with a poor prognosis and is a common primary brain tumor in adults, accounting for approximately 49% of malignant brain/CNS tumors [
1,
2]. Current glioblastoma management involves surgery followed by weeks of radiotherapy and concomitant daily temozolomide [
3]. While this approach once doubled the two-year survival rate for glioblastoma patients, glioblastoma’s prognosis remains very poor; its low survival rate, with only 5.5% of patients surviving five years post-diagnosis [
4], creates a necessity for not only more research on current efforts in the management of the disease but for a widening of scope involving the search for efficient and efficacious alternatives or complements. Steps in this direction include the investigation of metformin (MET) and resveratrol (RES)—due to their displays of anticancer activities—and their effects on major metabolic, proliferative, and apoptotic pathways involved in glioblastoma.
Phytochemicals, bioactive plant-derivatives, exert many health-promoting effects, including antioxidant, genoprotective, antineoplastic, anti-inflammatory, and antiangiogenic efficacy in various cancer types [
5,
6]. They also have a role in carcinogenesis by modulating miRNA expression, which regulates tumors by acting as tumor suppressors or oncogenes [
7]. Metformin (1,1-dimethylbiguanide) is a synthetic derivative of galegine and/or guanidine originating from the plant
Galega officinalis or French lilac [
8]. It is an FDA-approved antidiabetic drug—the first-line treatment of type 2 diabetes mellitus—and is widely studied. It shows potential against various cancers and also exhibits antineoplastic effects in brain tumors specifically [
9]. Resveratrol (3, 5, 4′-trihydroxystilbene) is a nonflavonoid polyphenol that is found in grapes, peanuts, and other plant sources. It has various therapeutic physiological effects on the human body, ranging from cardioprotective and antidiabetic effects to antioxidant potential and antitumor effects [
10].
Hyperglycemia, a defining symptom of diabetes mellitus, is a risk factor for some cancers, including gliomas [
11]. Both metformin and resveratrol have promising anticancer effects because they decrease the development of some cancer cell lines in in vitro studies through pathways, including the PI3K-Akt, AMPK-mTOR, and MAPK cascades [
12,
13,
14,
15]. Reducing proliferation and inducing apoptosis through these pathways cause a decrease in cell development and, thus, reduce cancer progression and symptoms.
2. Metformin and Resveratrol on Glioblastoma’s Proliferative and Apoptotic Pathways
2.1. PI3K/Akt Pathway
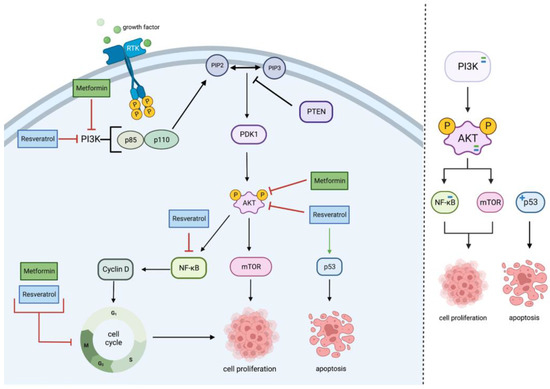
The PI3K/Akt signaling pathway is significant in cancer therapy as its activation results in downstream proteins and pathways that enhance cell proliferation and survival. MET inhibits the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathway when applied to glioblastoma cell lines, primarily by inhibiting PI3K or phosphorylated Akt. Inhibition of this pathway results in a decrease in the cellular invasion, migration, cell survival, and cell proliferation [
16,
17,
18]. Additionally, it inhibits the cell cycle by arresting cells in the G1, S, and M phases and increasing the number of cells in the G0 phase preceding apoptosis induction [
18].
RES, like MET, downregulates the PI3K/Akt and NF-κB pathways by decreasing the expression of PI3K class III, p-Akt, NF-κB, and miR-21. Consequently, cell proliferation, invasion, migration, and autophagy are diminished [
19,
20,
21,
22,
23,
24]. RES increases p53 expression, which increases apoptosis in U87 cell lines, specifically through SIRT1-dependent apoptosis [
19,
21,
22]. An S-G2/M cell cycle arrest is also observed when glioblastoma cell lines are treated with resveratrol [
23].
MET and RES inhibit PI3K/Akt signaling primarily by inhibiting the two PI3K phosphorylated subunits, the regulatory domain p85 and the catalytic domain p110, triggering a cascade of events that inhibits PIP2, PIP3, and PDK1, which are usually found to be upregulated in cancers as seen in
Figure 1 [
25,
26,
27,
28,
29].
Figure 1. Metformin and resveratrol’s action on PI3K/Akt pathway. Metformin and resveratrol inhibit PI3K and Akt, decreasing cell proliferation. Resveratrol decreases NF−κB expression, resulting in cell cycle arrest and decreased cell proliferation. Metformin and resveratrol also arrest the cell cycle directly. Resveratrol upregulates p53, increasing apoptosis. Generated using BioRender.
2.2. mTOR Pathway
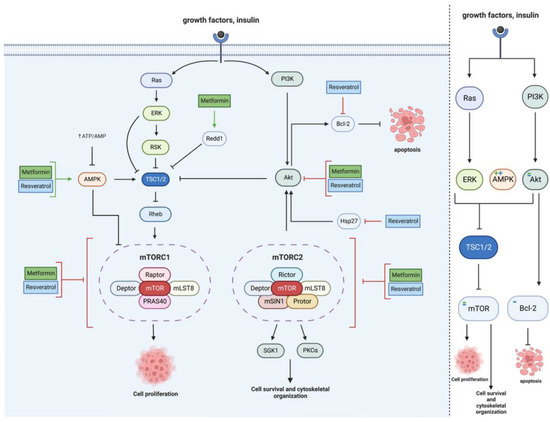
The target of rapamycin (mTOR) is crucial in several signaling pathways involved in glioblastoma cell growth, proliferation, and survival, making mTOR an exciting target for drugs such as MET and RES. It involves two complexes—mTORC1 (modulates growth and metabolism) and mTORC2 (modulates cell proliferation and survival)—and, when upregulated, it leads to tumor progression [
30]. MET decreases mTOR expression alongside increased AMPK expression (an mTOR inhibitor) in human glioma cells [
31].
RES shows similar effects of increased apoptosis and caspase activity through mTOR pathways. Additionally, it effectively synergized with temozolomide (TMZ) in SHG44 cells to inhibit mTOR through the AMPK/mTOR pathway, where the combination of TMZ and RES had a significantly greater inhibitory effect on mTOR phosphorylation than TMZ alone [
34].
Due to mTOR’s vast signaling reach, the effects of MET and RES on the mTOR pathway occur primarily through the effects of MET and RES on signaling intermediates, including Akt, Hsp27, AMPK, Redd1, TSC1/2, and Bcl-2, as seen in Figure 2.
Figure 2. Metformin and resveratrol’s action on mTOR. Metformin and resveratrol inhibit Akt and activate AMPK, activating TSC1/2 and inhibiting mTOR and cell proliferation. Metformin increases Redd1 expression, activating TSC1/2 and reducing cell proliferation. Resveratrol decreases Hsp27 expression, which decreases Akt activation. Resveratrol also increases Bcl−2, which increases apoptosis. Metformin and resveratrol inhibit mTOR, decreasing cell proliferation, survival, and cytoskeletal organization. Generated using BioRender.
2.3. RAS/RAF/MAPK Pathway
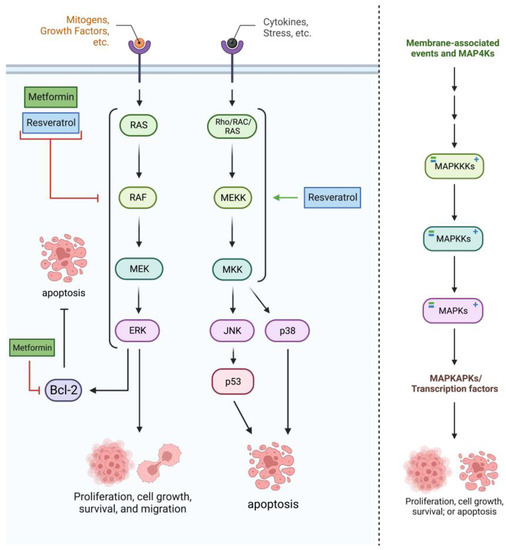
The mitogen-activated protein kinase (MAPK) signaling pathway constitutes a kinase cascade with many signaling proteins to target and regulate. Treatment with MET downregulates this pathway at many points, decreasing the expression of the MAP4K RAF, the MAP3K RAS, the MAP2K MEK-1, and the MAPK ERK-1 [
36]. This decreased expression comes alongside decreased antiapoptotic Bcl-2. Another study on glioblastoma stem cells (GSC) suggests that MET induces autophagy and apoptosis by stimulating the MAPK pathways instead, likely through the MAPKs p38 and JNK [
37].
Treatment with RES induces activation of the MAPK subfamily—including p-ERK, p-p38, and p-JNK—through ROS generation, inducing apoptosis as seen in
Figure 3 [
38]. This activation of the MAPK subfamily aligns with studies in which RES treatment activated p38-MAPK and thus increased autophagy rates in other cancer types [
29]. Although not in glioblastoma cells specifically, RES also seems to block the MAPK pathway to induce apoptosis, likely through the pathway’s proliferative distributaries (such as the ERK1/2 cascade). Note that the MAPKs interact with many downstream proteins, and MET and RES’s effects on this pathway could be carried out through factors such as HSF1, Hsp27, Hsp70, and more [
22].
Figure 3. Metformin and resveratrol’s interactions with the mitogen-activated protein kinase (MAPK) pathway. Primarily, metformin and resveratrol inhibit the MAPK/ERK cascade, decreasing levels of MAPK4K’s (RAF), MAP3K’s (RAF), MAP2K’s (MEK1/2), MAPK’s (ERK1/2), and associated proteins (Bcl−2). Resveratrol activates MAPK’s, including p−38 and JNK. Generated using BioRender.
2.4. AMPK Pathway
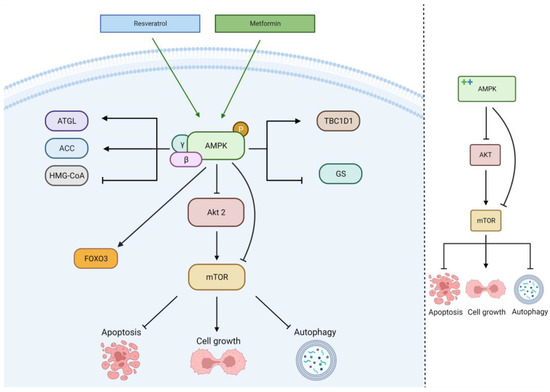
When activated by MET or RES, the AMP-activated protein kinase (AMPK) signaling pathway hinders viability and proliferation in glioblastoma cell lines. MET primarily activates the AMPK signaling pathway, increasing the intracellular AMP to ATP ratio [
39,
40]. MET also activates FOXO3 through AMPK activation, eliminating glioma-initiating cells [
41].
The activation of the AMPK signaling pathway by MET or RES leads to several effects on lipid and glucose metabolism: increased fatty acid catabolism through adipose triglyceride lipase (ATGL), regulation of fatty acid synthesis/oxidation through regulation of acetyl-CoA carboxylase (ACC), reduction of cholesterol synthesis through the reduction of HMG-CoA activity, inhibition of glycogenesis (GS), and the regulation of glucose uptake and glycolysis through TBC1D1, as seen in
Figure 4 [
40,
43,
44]. These effects, alongside other mTOR-mediated effects, improve glioblastoma cell metabolic programming, suppressing tumor growth.
Figure 4. Metformin and resveratrol’s action on AMPK pathway. Metformin and resveratrol activate AMPK and FOXO3, downregulating Akt, mTOR, and cell growth. AMPK regulates HMG−CoA, GS, ATGL, ACC, and TBC1D1. Generated using BioRender.
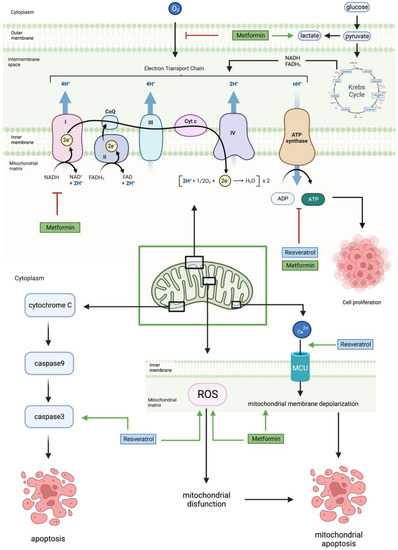
2.5. Mitochondrial Pathway
Mitochondria are the powerhouses of the cell, involved in ATP production, proliferation, apoptosis, and calcium homeostasis in glioblastoma cells. MET reduces oxygen consumption and the activity of the electron transport chain complex I, thereby decreasing mitochondrial ATP production [
18,
45]. MET reduces membrane potential, mitochondrial transcription factor A (mtTFA), and coactivator PGC-1a, thus decreasing mitochondrial biogenesis [
46].
Figure 5 shows how RES decreases mitochondrial ATP production and results in mitochondrial apoptosis and cell sensitivity by increasing caspase-3 activity, ROS production, and calcium ion influx [
45,
49]. RES also induces mitochondrial apoptosis by causing the collapse of the mitochondrial membrane potential, much like MET [
50].
Figure 5. Metformin and resveratrol’s action on the mitochondria. Metformin increases lactate production, decreases oxygen feeding into the ETC, and inhibits complex 1 of the ETC. Additionally, metformin and resveratrol decrease ATP levels, ultimately reducing cell proliferation. Metformin increases mitochondrial membrane depolarization and ROS levels, causing mitochondrial dysfunction and mitochondrial apoptosis. Resveratrol increases ROS levels and the influx of calcium ions into the mitochondria, causing mitochondrial membrane depolarization, which leads to mitochondrial apoptosis, and increases caspase−3 levels, leading to apoptosis. Generated using BioRender.
2.6. In Vivo
In vivo studies on xenograft mice models involved injecting them with glioblastoma cell lines and treating them with MET or RES. RES also decreases tumor growth, volume, and the Ki-67 staining index and increases apoptosis, autophagy, and model survival. RES decreased Bcl-2, EGFR, NF-κB, COX-2, and VEGF levels [
21,
34,
51].
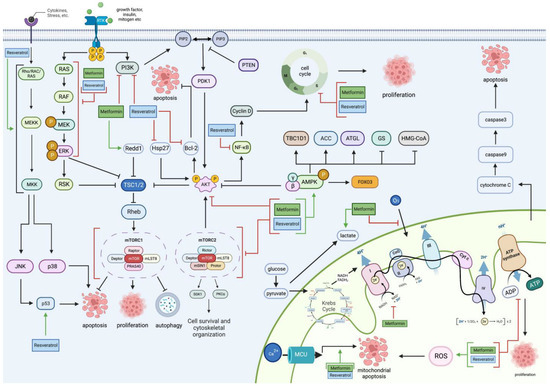
The complete molecular pathway targeted at different locations by both MET and RES is seen in Figure 6.
Figure 6. An overview of metformin and resveratrol’s actions on glioblastoma. Metformin and resveratrol inhibit PI3K, Akt, and the MAPK/ERK pathway and increase AMPK. Consequently, they increase TSC1/2, decrease mTOR, cell proliferation, cell survival, and cytoskeletal organization, and trigger apoptosis and autophagy. Metformin also increases Redd1 levels, which increases TSC1/2. Resveratrol decreases Hsp27 and Bcl-2 and increases p53 and MAPKs, which include JNK and p38, increasing apoptosis and decreasing NF-κB, inhibiting the cell cycle and cell proliferation. Metformin and resveratrol both inhibit the cell cycle halting cell proliferation. Metformin inhibits the electron transport chain by increasing lactate production, decreasing oxygen levels fed into the electron transport chain, and inhibiting complex 1. Metformin and resveratrol decrease ATP levels, decreasing cell proliferation. Resveratrol and metformin increase ROS levels, which cause mitochondrial apoptosis associated with mitochondrial membrane depolarization. Resveratrol increases caspase-3 levels, leading to apoptosis. Generated using BioRender.
3. Metformin and Resveratrol on Glucose in Glioblastoma
The interaction of glucose with different pathways, such as the nuclear factor kappa beta (NF-κB) pathway and glycolytic pathway, possibly increased cell proliferation, cell viability, tumorigenesis, NF-κB phosphorylation, and the expression of Bcl-2, Mcl-1, FPR1, EGFR, VEGF, ERK, EGF, ROS production, STAT3, PDK1, PDK3, ECH, and HADH [11,52,53,54]. These effects arose through multiple signaling intermediates and interactions. For example, high glucose upregulates the expression of a G-protein coupled chemoattractant receptor (GPCR), formyl peptide receptor 1 (FPR1), and epidermal growth factor receptor (EGFR) on GBM cells [11].
A high glucose-induced increase in the Warburg effect is also observed: where cancer cells alter molecular pathways and switch from oxidative phosphorylation to aerobic glycolysis [5,53]. This alteration—the Warburg phenotype—then composes numerous selective molecular advantages for the growth and proliferation of tumor cells.
4. Clinical Considerations and Relevance
4.1. Bioavailibility
Despite their common usage, metformin is associated with low gastrointestinal absorption (40–60%) and bioavailability upon oral administration [
66]. This naturally necessitates higher dose administrations and/or dose frequencies and could increase dose-related side effects, not to forget negatively affecting patient compliance. Steady-state plasma levels of metformin have been reported to range from 10 µmol/L to 40 µmol/L; a 850 mg dose thrice daily (with standard doses ranging from 500 to 2550 mg daily) leads to steady-state plasma concentrations of around 1.35 mg/L in both healthy and diabetic patients [
67,
68].
Resveratrol, when taken orally, is absorbed well with a bioavailability of around 70%; however, the availability of RES itself is minimal [
69]. This is due to extensive liver and intestines metabolism that produces lower activity metabolites than RES through glucuronidation and sulfation [
70].
4.2. Delivery
To combat the drugs’ low bioavailability, decrease adverse reactions and metabolism, and increase targeting to the brain, several novel drug delivery methods of metformin and resveratrol have been studied. Since transport through the BBB depends on the lipid solubility of substances and phytochemicals, these methods involve lipid-designed drug delivery systems, nanotechnologies, and encapsulation methods [
75].
To enhance delivery to the bloodstream and tissues in general, surface-modified nanostructured lipid carriers (PEGylated NLCs) have shown promise in improving metformin’s release, delivery, and bioavailability in in vivo enhancing pharmacokinetics, even at reduced doses [
66]. Similarly, lipid nanocarriers, nanocrystals, and other technologies enhanced the in vivo pharmacokinetic profile and delivery of resveratrol [
69,
76,
77].
4.3. Clinical Trials
There are very few clinical studies on metformin in glioblastoma, and none on resveratrol, despite the promising in vitro and in vivo research.
A phase I clinical study investigating the use of metformin with TMZ in newly diagnosed glioblastoma patients found that doses of 2250 mg of metformin per day were tolerable, with no dose-limiting toxicities, although manageable adverse effects included appetite loss, nausea, and diarrhea [
85].
5. Conclusions
Metformin and resveratrol antidiabetic drugs show antineoplastic mechanisms against glioblastoma cells by increasing apoptosis and autophagy and decreasing proliferation by altering the PI3K/Akt, mTOR, AMPK, MAPK, and mitochondrial pathways. Alongside heavily affecting cancer cell glucose metabolism with potent effects in vitro and in vivo, and having new delivery mechanisms that ameliorate their poor bioavailability, metformin and resveratrol show promise in glioblastoma treatment. The large pool of evidence warrants further clinical research to profile the drugs’ pharmacokinetics in glioblastoma patients and investigate the effectiveness of metformin and resveratrol as separate or combined complements to the current glioblastoma treatments of chemotherapy or radiotherapy. As there is currently no literature available on the combined effects of metformin and resveratrol on glioblastoma cells, the combination of metformin and resveratrol treatment on GBM cell cultures must be investigated in vitro by employing varying concentrations of both metformin and resveratrol, and comparing these to a resveratrol-only control, a metformin-only control, and proper control. Appropriate analyses (viability assays, migration assays, gene expression analyses, and cell cycle analyses) must then be undertaken to confirm or deny the possibility of synergy between metformin and resveratrol on glioblastoma cells. Based on the results of the in vitro investigations, possible in vivo studies and clinical trials (phase I, non-inferiority, etc.) can be duly planned and executed. Such studies on glioblastoma cells and patients may lead to the discovery of a more effective and powerful therapy that can be implemented in future treatments.
This entry is adapted from the peer-reviewed paper 10.3390/cancers15133368