1. Introduction
Nanomaterials are ideal candidates for the analysis of plant pathogens as their dimensions typically fall in the range of 1 to 100 nm, which can provide enhanced surface-to-volume ratio and unique chemical, optical, and electrical properties, which are not observed in the bulk counterparts [
123]. Nanomaterials are normally synthesized via specific self-assembly procedures at the nanoscale level [
124].
Nanoscale materials can also interact with biomolecular targets more efficiently due to their small sizes and fast diffusion rates [
123]. Nanomaterials, such as carbon-based materials, such as graphene and carbon nanotubes, including graphite, silver (Ag), gold (Au), silica (Si), and copper (Cu) nanoparticles [
125], can be prepared in many different morphologies ranging from spherical particles, cubes, rods, wires, plates, prisms, and core–shell structures to more complicated 3D architectures. In addition, new advanced nanomaterials, such as nanotubes, nanorods, nanospheres, nanoreefs, nanoclusters, tetrapods, nanopores, and nanochannels, have been created as a result of the growth of nanotechnology as sensing tools [
126], which have been widely applied in the areas of in vivo imaging [
127,
128,
129]. These could undergo shape transformation or agglomeration, which alters their chemical or physical properties in response to various external stimuli [
130].
Biosensor devices are derived by their bioinspired receptor units with differently efficient specificities to their targeted analytes [
131]. For example, enzymes can react with certain molecules rapidly and selectively, and nucleic acids can bind to their complementary sequences delicately on the nanoscale [
132]. The biorecognition components may consist of enzymes, nucleic acids, entire cells, aptamers, or polymers, whereas the analyte may be an ion, molecule, cell, nucleic acid, or protein [
133]. The addition of nanomaterials (NMs) into a biosensor can detect the signals and provide a more accurate and specific identification of an analyte [
134,
135]. For example, carbon nanomaterials are suitable for the conjugation of biomolecules (enzymes, antibodies, DNA, cell, etc.) [
132]. In addition, biomolecules can be immobilized and conjugated with other materials by surface modification through the recombination or introduction of chemical linkers [
136]. In biotechnology-derived sensing techniques, gold nanoparticles (AuNPs) are commonly used; they can be used as carriers in the case of biorecognition molecules, antibodies, or aptamers, and can also be the labels for signal transduction and amplification [
137]. For example, absorption-based colorimetric sensing involves aggregation-induced interparticle surface plasmon coupling of AuNPs, which results in a visible color change from red to blue. This concept has provided a practical platform for the detection of any target analyte that triggers the AuNPs’ aggregation or redispersion [
138]. AuNPs are widely utilized in electrochemical-based biosensors as they can increase the surface area and conductivity and have been reported in the detection of microbial food pathogens [
139].
These advanced nanomaterials have recently begun to play a crucial role in the creation of biosensors, tissue engineering, battery materials, and multifunctional materials [
127,
140]. The three main parts of a biosensor are (i) a biorecognition element for detecting the presence and concentration of an analyte; (ii) an optical, electrochemical, piezoelectric, or thermally based transducer surface; and (iii) a detector to measure the signals produced [
141].
The sensing applications would be a fruitful strategy for developing a novel tool of diagnostics, especially for Alternaria.
2. Colorimetric Sensing
Colorimetric sensors are based on simple chemical reaction principles and have become more usable and widespread due to their vast availability, simple use, inexpensiveness, and ability to respond sensitively and selectively to a variety of analytes. Colorimetric sensors are one of the types of optical sensors; they change color in response to external inputs if any modification to the physical or chemical surroundings qualifies as such a stimulus [
142]. Four examples of colorimetric techniques include “indirect target-mediated aggregation, chromogenic substrate-mediated catalytic activity, point-of-care testing (POCT) devices, and machine learning-assisted colorimetric sensor arrays”, which can be utilized for pathogen analysis [
143,
144].
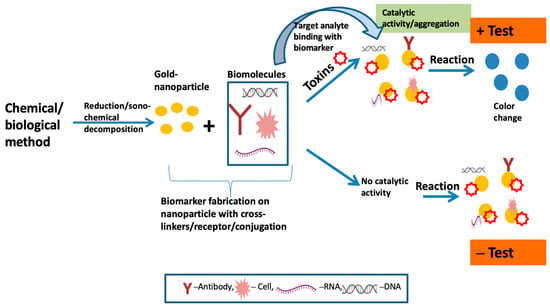
Figure 1 depicts a pictorial general working overview of colorimetric-based biosensors with the fabrication of AuNPs to identify the target analytes in samples. To attain mobility and intellectualization in clinical applications, colorimetric-sensor-based gold nanoparticles can be further integrated with newly developed portable devices, such as paper-based chips. This can be applied to smartphone readout applications and can help with the visualization and in situ detection of germs [
69].
Figure 1. Schematic diagram of colorimetric-based biosensor for analyte detection.
3. Paper-Based Sensing
Paper is a versatile material and gaining attention as a substrate for developing paper-based analytical devices (PADs) in point-of-care (POC) format, being an alternative to the detection of conventional biomarkers [
145]. Due to its porous nature, the paper has an inherent hydrophilic feature that is particularly useful since it may promote the flowing of fluid via capillary reaction without external pumping [
146]. PADs are less expensive and simpler to make than sensors composed of materials such as glass, silicon, and plastic [
147]. Additionally, PADs can be disposed of in an environmentally friendly manner and are simple to store, transport, and store. Three types of PADs include dipstick assay, lateral flow assay (LFA), and microfluidic paper-based analytical device [
148]. Membranes made of cellulose and nitrocellulose are the two main forms of paper substrates.
Paper-derived colorimetric sensors are able to use enzyme-based reactions and have the ability for real-field diagnosis. The sample is put onto a test strip, and the result is displayed within 5 to 10 minutes, using the paper-based lateral flow immunochromatographic analysis test, which analyzes the quality and quantity of the material in a complicated mixture [
75]. A paper-based colorimetric sensor was used to predict plant diseases in
Allium crops for the easy and simple measurement of PG activity with the help of the principle of the ruthenium red (RR) dye method [
149]; however, the drawback of hindrance can be developed by blurry signaling at the detection point due to capillary reaction forces on the liquid and its corresponding membrane [
150].
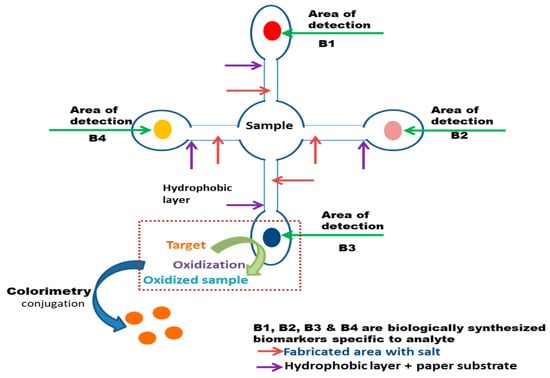
Figure 2 illustrates the fundamental functioning of a microfluid paper-based biosensor, implemented with the colorimetric methodology for the observation of color change in case of any targeted toxins detection.
Figure 2. Representation of a basic paper-based biosensor via microfluid mechanism for the target.
4. Surface Plasmon Resonance (SPR)-Based Biosensing
SPR-derived sensors are suitable for the measurement of biological molecular associations in real time, and are helpful analytical tools for calculating the adsorption rate of target analytes onto molecularly derived probes connected with a metallic surface [
151]. The electromagnetic (EM) resonance of the group oscillations of free electrons connected to a plasmonic metal (silver or gold for visible spectrum) and a dielectric semi-infinite interface is known as surface plasmon resonance (SPR) [
152]. This resonance produces a linked, exponentially decaying surface EM field along the metal dielectric contact. This field can be employed as a sensing layer to implement SPR-based sensors since it is particularly sensitive to changes in the refractive index (RI) of the dielectric layer [
153]. The SPR-based biosensor was utilized for the detection of the fungus
P. fijiensis in samples of banana leaf extracts [
154]. However, plasmonic sensors also have many drawbacks, such as nonspecific surface binding, mass transportation restrictions, steric hindrance during the binding event, and data misinterpretation during common events [
88]. Localized surface plasmon resonance (LSPR) is another promising method related to SPR biosensing with high sensitivity, a type of SPR phenomenon in which the resonant EM field is constrained to the metallic nanostructure and sensitive to RI change of the medium surrounding it only within a few tens of nm [
155]. For colloidal and randomly oriented nanoparticles, scattering and absorption effects are dominant. The advantages of LSPR over SPR include a high aspect ratio that permits a larger interaction surface area for immobilizing the sensing elements, a miniaturized probe to create compact devices, and broad applicability and compatibility with a variety of phenomena, including fluorescence, Raman, and IR spectroscopy, among others less perceptive than traditional SPR [
156,
157].
5. Chip-Based Rapid Sensing
In chip-based biosensors, the sensing layer is created over a thin metal (50 nm)–coated glass substrate, and the analyte to be recognized flows through a microfluidic channel near the sensing layer [
153]. As mentioned, these chip-based sensors have many benefits, including label-free detection, dynamic measurement of binding–unbinding kinetics to watch the reaction mechanism occurring over the sensing surface, and high sensitivity [
158]. Microfluidics, for example, can be integrated with chip-based substrates that are highly sensitive, repeatable, and miniaturized [
155]. Chip-based biosensors are appropriate for microfluidics-based methods, which allow mixing, filtering, and detection to be carried out on the same chip by adjusting tiny amounts of liquid. The DNA of
Ganoderma boninense, a pathogenic fungus that infects oil palm trees in Malaysia, was extracted using a microfluidic chip device [
159]. The increasing research and development in this area have demonstrated its potential for real-time evaluation and mobility, as well as prospective future works in terms of the improvement of multiple biosensors and multiple pathogen detection in a single-chip platform [
159,
160].
6. Electrochemical Sensing
In electrochemical-based biosensors, a molecular recognition coating and an electrochemical transducer, which transforms biological data obtained from a connecting event into an electrical signal and then displays them on a readout device, are two main components [
161]. Different types and forms of nanomaterials and nanocomposites could be used in electrochemical biosensors to improve the sensitivity of the detection mechanisms and to give better detection limits through various tactics [
126,
162]. Microfluidic systems and electrochemical biosensors can be integrated to provide miniaturized components on a single platform [
163]. The urediniospores of soybean rust fungi were detected with aptamer-based sensitive electrochemical sensing. For this, spore-bound aptamer units were incubated with a streptavidin–alkaline phosphatase agent, leading to the enzymatic formation of p-nitrophenol, which is characterized by its unique electrochemical properties [
164]. Regarding the electrochemical nano-biosensor of bacterial species, recently, an electrochemical-based immune sensor was manufactured for the detection of
Escherichia coli O157:H7 (
E. coli O157:H7) using cyclic voltammetry (CV) with the combination of nanomaterials [
143,
165]. An electrochemical-derived impedance immune sensor for the detection of strained
Staphylococcus aureus was also introduced [
166].
Electrochemical methods, such as electrochemical impedance spectroscopy (EIS), differential pulse voltammetry (DPV), cyclic voltammetry (CV), square wave voltammetry (SWV), and amperometry, have been implemented for pathogen detection and identification [
167]. Nanomaterials or polymers have been normally applied with these methods to improve signals.
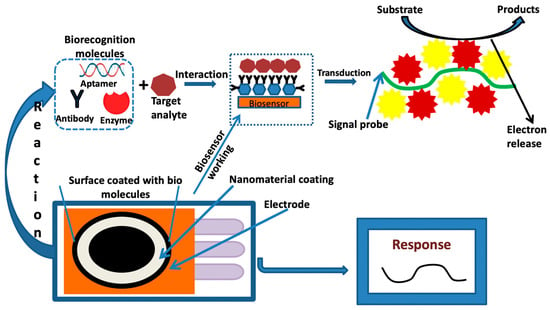
Figure 3 defines the fundamental principle of the electrochemical-based nanosensor techniques for any kind of desired analyte.
Figure 3. Pictorial representation of the fundamental process of the electrochemical-based nanosensor.
Electrochemical-based platforms are the most successful biosensors, and they have been used for the detection and identification of huge numbers of biomarkers and the diagnosis of infectious diseases, including cancers [
168]. Despite the advantages of electrochemical-based biosensors, there are a few problems associated, such as analyte detection and analyte loss during the transporting of the sample to the electrode surface [
169,
170]. By assessing the different applications and utility of these sensing techniques with plant pathogens, it was discovered that they are fast, sensitive, accurate, specific, and suitable for their portability. This can be applied with slight modifications to manufacture an efficient tool for the detection of
Alternaria.
This entry is adapted from the peer-reviewed paper 10.3390/bios13070701