SARS-CoV-2, a single-stranded RNA coronavirus, causes an illness known as coronavirus disease 2019 (COVID-19). Long-term complications are an increasing issue in patients who have been infected with COVID-19 and may be a result of persistent viral-associated systemic and central nervous system inflammation or may be attributed to virus-induced microvascular blood clots. COVID-19 may incite changes in brain function with a wide range of lingering symptoms. Patients often experience fatigue and may note brain fog, sensorimotor symptoms, headaches and sleep disturbances. Prolonged neurological and neuropsychiatric symptoms are prevalent and can interfere substantially in everyday life, leading to a massive public health concern.
- COVID-19
- long COVID syndrome
- brain fog
- fatigue
- myalgia
1. Fatigue
2. Neuropsychiatric Sequelae
3. Sleep Disorders
4. Sensorimotor Deficits
4.1. Prevalence and Spectrum of Symptoms
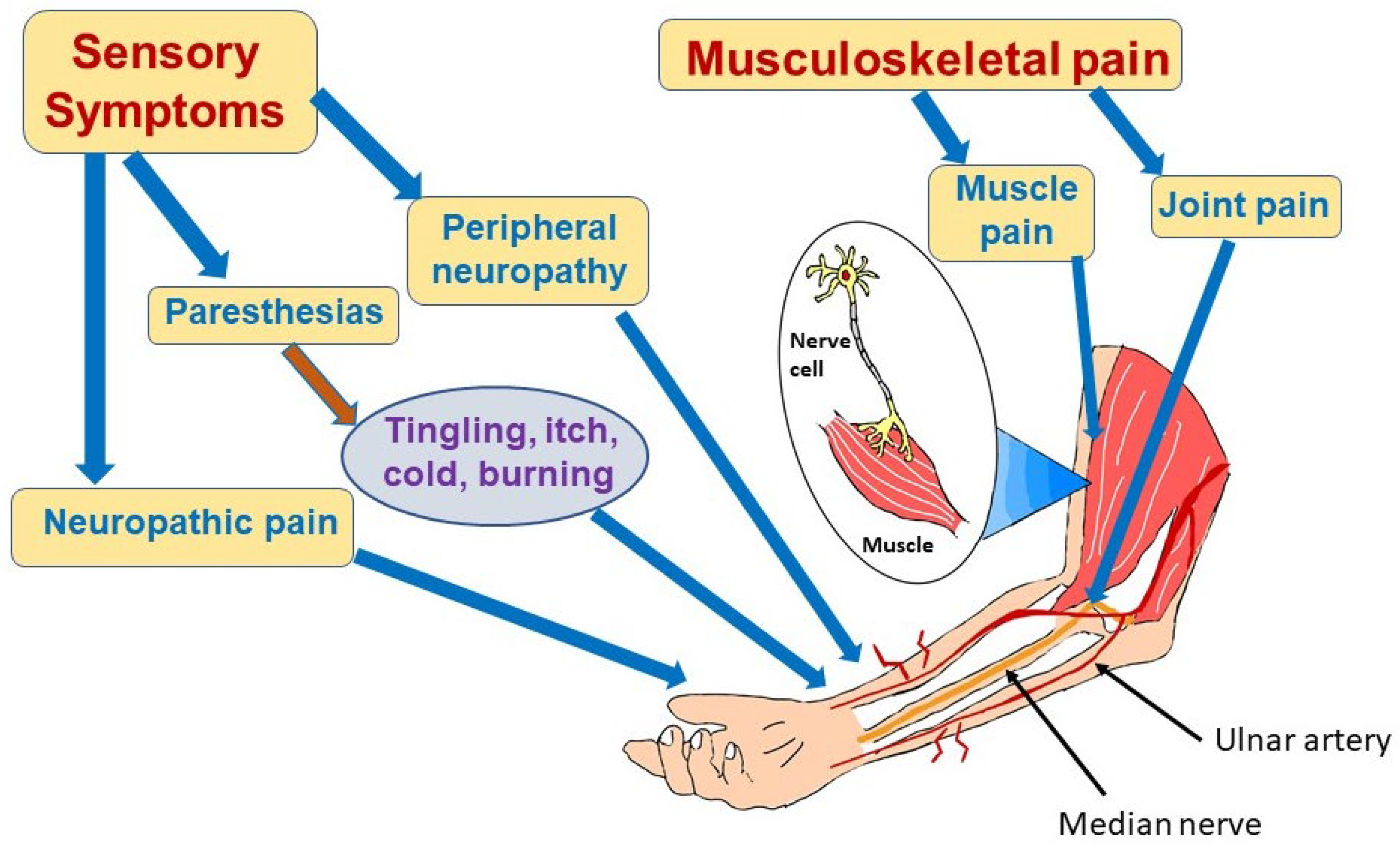
4.2. COVID-19-Associated Neuropathic Pain and Neuropathies
4.3. Myalgias
4.4. Pathophysiology of Long COVID Effects on the Peripheral Nerves
5. Cognitive Impairment and Brain Fog
6. Hyposmia, Hypogeusia, Hearing Loss
7. Ocular Symptoms
8. Conclusions
Long COVID-associated neurocognitive impairment is a serious public health concern. It can affect those with mild or severe initial infection and spans all age groups. Persons with long COVID may find their quality of life compromised and their productivity reduced. Fatigue and brain fog are common. Anxiety, depression, poor sleep quality, and myalgias can add to the quality of life issues. There are no proven effective treatments for the multitude of neuropsychiatric symptoms of long COVID. A multi-pronged approach is often desirable and addresses the array of symptoms to support recovery through rehabilitation, good nutrition and psychiatric care. An individualized care plan can yield maximum benefit. More research is clearly needed to improve our understanding of the etiology of long COVID and provide a road map toward effective therapies.
This entry is adapted from the peer-reviewed paper 10.3390/neurolint15030052
References
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e0240784.
- Healey, Q.; Sheikh, A.; Daines, L.; Vasileiou, E. Symptoms and signs of long COVID: A rapid review and meta-analysis. J. Glob. Health 2022, 12, 05014.
- Aucott, J.N.; Rebman, A.W. Long-haul COVID: Heed the lessons from other infection-triggered illnesses. Lancet. 2021, 397, 967–968.
- El Sayed, S.; Shokry, D.; Gomaa, S.M. Post-COVID-19 fatigue and anhedonia: A cross-sectional study and their correlation to post-recovery period. Neuropsychopharmacol. Rep. 2021, 41, 50–55.
- Mudgal, S.K.; Gaur, R.; Rulaniya, S.; Latha, T.; Agarwal, R.; Kumar, S.; Varshney, S.; Sharma, S.; Bhattacharya, S.; Kalyani, V. Pooled Prevalence of Long COVID-19 Symptoms at 12 Months and Above Follow-Up Period: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e36325.
- Logue, J.K.; Franko, N.M.; McCulloch, D.J.; McDonald, D.; Magedson, A.; Wolf, C.R.; Chu, H.Y. Sequelae in adults at 6 months after COVID-19 infection. JAMA Network Open 2021, 4, e210830.
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019.
- Verveen, A.; Wynberg, E.; van Willigen, H.D.G.; Boyd, A.; de Jong, M.D.; de Bree, G.; Davidovich, U.; Lok, A.; Moll van Charante, E.P.; Knoop, H.; et al. Severe Fatigue in the First Year Following SARS-CoV-2 Infection: A Prospective Cohort Study. Open Forum Infect. Dis. 2022, 9, ofac127.
- Stefanou, M.I.; Palaiodimou, L.; Bakola, E.; Smyrnis, N.; Papadopoulou, M.; Paraskevas, G.P.; Rizos, E.; Boutati, E.; Grigoriadis, N.; Krogias, C.; et al. Neurological manifestations of long-COVID syndrome: A narrative review. Ther. Adv. Chronic Dis. 2022, 13, 20406223221076890.
- Harenwall, S.; Heywood-Everett, S.; Henderson, R.; Godsell, S.; Jordan, S.; Moore, A.; Philpot, U.; Shepherd, K.; Smith, J.; Bland, A.R. Post-Covid-19 Syndrome: Improvements in Health-Related Quality of Life Following Psychology-Led Interdisciplinary Virtual Rehabilitation. J. Prim. Care Community Health 2021, 12, 21501319211067674.
- Azzolino, D.; Cesari, M. Fatigue in the COVID-19 pandemic. Lancet Healthy Longev. 2022, 3, e128–e129.
- Ladds, E.; Rushforth, A.; Wieringa, S.; Taylor, S.; Rayner, C.; Husain, L.; Greenhalgh, T. Persistent symptoms after COVID-19: Qualitative study of 114 “long covid” patients and draft quality principles for services. BMC Health Serv. Res. 2020, 20, 1144.
- Rass, V.; Ianosi, B.A.; Zamarian, L.; Beer, R.; Sahanic, S.; Lindner, A.; Kofler, M.; Schiefecker, A.J.; Mahlknecht, P.; Heim, B.; et al. Factors associated with impaired quality of life three months after being diagnosed with COVID-19. Qual. Life Res. 2022, 31, 1401–1414.
- Tabacof, L.; Tosto-Mancuso, J.; Wood, J.; Cortes, M.; Kontorovich, A.; McCarthy, D.; Rizk, D.; Rozanski, G.; Breyman, E.; Nasr, L.; et al. Post-acute COVID-19 Syndrome Negatively Impacts Physical Function, Cognitive Function, Health-Related Quality of Life, and Participation. Am. J. Phys. Med. Rehabil. 2022, 101, 48–52.
- Twomey, R.; DeMars, J.; Franklin, K.; Culos-Reed, S.N.; Weatherald, J.; Wrightson, J.G. Chronic Fatigue and Postexertional Malaise in People Living With Long COVID: An Observational Study. Phys. Ther. 2022, 102, pzac005.
- Spudich, S.; Nath, A. Nervous system consequences of COVID-19. Science 2022, 375, 267–269.
- Mackay, A. A Paradigm for Post-Covid-19 Fatigue Syndrome Analogous to ME/CFS. Front. Neurol. 2021, 12, 701419.
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135.
- Boldrini, M.; Canoll, P.D.; Klein, R.S. How COVID-19 Affects the Brain. JAMA Psychiatry 2021, 78, 682–683.
- Rudroff, T.; Fietsam, A.C.; Deters, J.R.; Bryant, A.D.; Kamholz, J. Post-covid-19 fatigue: Potential contributing factors. Brain Sci. 2020, 10, 1012.
- Ortelli, P.; Ferrazzoli, D.; Sebastianelli, L.; Maestri, R.; Dezi, S.; Spampinato, D.; Saltuari, L.; Alibardi, A.; Engl, M.; Kofler, M.; et al. Altered motor cortex physiology and dysexecutive syndrome in patients with fatigue and cognitive difficulties after mild COVID-19. Eur. J. Neurol. 2022, 29, 1652–1662.
- Versace, V.; Sebastianelli, L.; Ferrazzoli, D.; Romanello, R.; Ortelli, P.; Saltuari, L.; D’Acunto, A.; Porrazzini, F.; Ajello, V.; Oliviero, A.; et al. Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID-19. Clin. Neurophysiol. 2021, 132, 1138–1143.
- Sadlier, C.; Albrich, W.C.; Neogi, U.; Lunjani, N.; Horgan, M.; O’Toole, P.W.; O’Mahony, L. Metabolic rewiring and serotonin depletion in patients with postacute sequelae of COVID-19. Allergy 2022, 77, 1623–1625.
- Eroğlu, İ.; Eroğlu, B.Ç.; Güven, G.S. Altered tryptophan absorption and metabolism could underlie long-term symptoms in survivors of coronavirus disease 2019 (COVID-19). Nutrition 2021, 90, 111308.
- Calabria, M.; García-Sánchez, C.; Grunden, N.; Pons, C.; Arroyo, J.A.; Gómez-Anson, B.; Estévez García, M.; Belvís, R.; Morollón, N.; Vera Igual, J.; et al. Post-COVID-19 fatigue: The contribution of cognitive and neuropsychiatric symptoms. J. Neurol. 2022, 269, 3990–3999.
- Baslet, G.; Aybek, S.; Ducharme, S.; Modirrousta, M.; Nicholson, T.R. Neuropsychiatry’s Role in the Postacute Sequelae of COVID-19: Report From the American Neuropsychiatric Association Committee on Research. J. Neuropsychiatry Clin. Neurosci. 2022, 34, 341–350.
- Hejbøl, E.K.; Harbo, T.; Agergaard, J.; Madsen, L.B.; Pedersen, T.H.; Østergaard, L.J.; Andersen, H.; Schrøder, H.D.; Tankisi, H. Myopathy as a cause of fatigue in long-term post-COVID-19 symptoms: Evidence of skeletal muscle histopathology. Eur. J. Neurol. 2022, 29, 2832–2841.
- Pires, R.E.; Reis, I.G.N.; Waldolato, G.S.; Pires, D.D.; Bidolegui, F.; Giordano, V. What Do We Need to Know About Musculoskeletal Manifestations of COVID-19?: A Systematic Review. JBJS Rev. 2022, 10, e22.00013.
- Khraisat, B.; Toubasi, A.; AlZoubi, L.; Al-Sayegh, T.; Mansour, A. Meta-analysis of prevalence: The psychological sequelae among COVID-19 survivors. Int. J. Psychiatry Clin. Pract. 2021, 26, 234–243.
- Putri, C.; Arisa, J.; Hananto, J.E.; Hariyanto, T.I.; Kurniawan, A. Psychiatric sequelae in COVID-19 survivors: A narrative review. World J. Psychiatry 2021, 11, 821–829.
- Jackson, C.; Stewart, I.D.; Plekhanova, T.; Cunningham, P.S.; Hazel, A.L.; Al-Sheklly, B.; Aul, R.; Bolton, C.E.; Chalder, T.; Chalmers, J.D.; et al. Effects of sleep disturbance on dyspnoea and impaired lung function following hospital admission due to COVID-19 in the UK: A prospective multicentre cohort study. Lancet Respir. Med. 2023; Advance online publication.
- Zakia, H.; Pradana, K.; Iskandar, S. Risk factors for psychiatric symptoms in patients with long COVID: A systematic review. PLoS ONE 2023, 18, e0284075.
- Gramaglia, C.; Gattoni, E.; Gambaro, E.; Bellan, M.; Balbo, P.E.; Baricich, A.; Sainaghi, P.P.; Pirisi, M.; Binda, V.; Feggi, A.; et al. Anxiety, Stress and Depression in COVID-19 Survivors from an Italian Cohort of Hospitalized Patients: Results from a 1-Year Follow-Up. Front. Psychiatry 2022, 13, 862651.
- Alghamdi, H.Y.; Alrashed, A.M.; Jawhari, A.M.; Abdel-Moneim, A.S. Neuropsychiatric symptoms in post-COVID-19 long haulers. Acta Neuropsychiatr. 2022, 34, 318–329.
- Rass, V.; Beer, R.; Schiefecker, A.J.; Lindner, A.; Kofler, M.; Ianosi, B.A.; Mahlknecht, P.; Heim, B.; Peball, M.; Carbone, F.; et al. Neurological outcomes 1 year after COVID-19 diagnosis: A prospective longitudinal cohort study. Eur. J. Neurol. 2022, 29, 1685–1696.
- da Silva Lopes, L.; Silva, R.O.; de Sousa Lima, G.; de Araújo Costa, A.C.; Barros, D.F.; Silva-Néto, R.P. Is there a common pathophysiological mechanism between COVID-19 and depression? Acta Neurol. Belg. 2021, 121, 1117–1122.
- Matits, L.; Munk, M.; Bizjak, D.A.; Kolassa, I.T.; Karrasch, S.; Vollrath, S.; Jerg, A.; Steinacker, J.M. Inflammation and severity of depressive symptoms in physically active individuals after COVID-19—An exploratory immunopsychological study investigating the effect of inflammation on depressive symptom severity. Brain Behav. Immun. Health 2023, 30, 100614.
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427.
- Khan, S.; Karim, M.; Gupta, V.; Goel, H.; Jain, R. A Comprehensive Review of COVID-19-Associated Endocrine Manifestations. South. Med. J. 2023, 116, 350–354.
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Jubran, A.S.; Almulla, A.F.; Moustafa, S.R.; Maes, M. Increased insulin resistance due to Long COVID is associated with depressive symptoms and partly predicted by the inflammatory response during acute infection. Braz. J. Psychiatry 2023. Advance online publication.
- Bonati, M.; Campi, R.; Segre, G. Psychological impact of the quarantine during the COVID-19 pandemic on the general European adult population: A systematic review of the evidence. Epidemiol. Psychiatr. Sci. 2022, 31, e27.
- Dos Santos, E.R.R.; Silva de Paula, J.L.; Tardieux, F.M.; Costa-E-Silva, V.N.; Lal, A.; Leite, A.F.B. Association between COVID-19 and anxiety during social isolation: A systematic review. World J. Clin. Cases 2021, 9, 7433–7444.
- Pietrabissa, G.; Simpson, S.G. Psychological Consequences of Social Isolation During COVID-19 Outbreak. Front. Psychol. 2020, 11, 2201.
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid—Mechanisms, risk factors, and management. BMJ 2021, 374, n1648.
- Ahmed, G.K.; Khedr, E.M.; Hamad, D.A.; Meshref, T.S.; Hashem, M.M.; Aly, M.M. Long term impact of Covid-19 infection on sleep and mental health: A cross-sectional study. Psychiatry Res. 2021, 305, 114243.
- Jain, A.; Bodicherla, K.P.; Bashir, A.; Batchelder, E.; Jolly, T.S. COVID-19 and Obsessive-Compulsive Disorder: The Nightmare Just Got Real. Prim. Care Companion CNS Disord. 2021, 23, 20l02877.
- Loosen, A.M.; Skvortsova, V.; Hauser, T.U. Obsessive-compulsive symptoms and information seeking during the Covid-19 pandemic. Transl. Psychiatry 2021, 11, 309.
- Abba-Aji, A.; Li, D.; Hrabok, M.; Shalaby, R.; Gusnowski, A.; Vuong, W.; Surood, S.; Nkire, N.; Li, X.M.; Greenshaw, A.J.; et al. COVID-19 pandemic and mental health: Prevalence and correlates of new-onset obsessive-compulsive symptoms in a Canadian Province. Int. J. Environ. Res. Public Health 2020, 17, 6986.
- Linde, E.S.; Varga, T.V.; Clotworthy, A. Obsessive-Compulsive Disorder During the COVID-19 Pandemic-A Systematic Review. Front. Psychiatry 2022, 13, 806872.
- Kaseda, E.T.; Levine, A.J. Post-traumatic stress disorder: A differential diagnostic consideration for COVID-19 survivors. Clin. Neuropsychol. 2020, 34, 1498–1514.
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600.
- Mao, J.; Wang, C.; Teng, C.; Wang, M.; Zhou, S.; Zhao, K.; Ye, X.; Wang, Y. Prevalence and Associated Factors of PTSD Symptoms After the COVID-19 Epidemic Outbreak in an Online Survey in China: The Age and Gender Differences Matter. Neuropsychiatr. Dis. Treat. 2022, 18, 761–771.
- Greene, T.; El-Leithy, S.; Billings, J.; Albert, I.; Birch, J.; Campbell, M.; Ehntholt, K.; Fortune, L.; Gilbert, N.; Grey, N.; et al. Anticipating PTSD in severe COVID survivors: The case for screen-and-treat. Eur. J. Psychotraumatol. 2022, 13, 1959707.
- Houben-Wilke, S.; Goërtz, Y.M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.; van Herck, M.; Burtin, C.; Posthuma, R.; Franssen, F.M.; et al. The Impact of Long COVID-19 on Mental Health: Observational 6-Month Follow-Up Study. JMIR Ment. Health 2022, 9, e33704.
- Savarraj, J.P.J.; Burkett, A.B.; Hinds, S.N.; Paz, A.S.; Assing, A.; Juneja, S.; Colpo, G.D.; Torres, L.F.; Cho, S.M.; Gusdon, A.M.; et al. Pain and Other Neurological Symptoms Are Present at 3 Months After Hospitalization in COVID-19 Patients. Front. Pain Res. 2021, 2, 737961.
- Schou, T.M.; Joca, S.; Wegener, G.; Bay-Richter, C. Psychiatric and neuropsychiatric sequelae of COVID-19—A systematic review. Brain Behav. Immun. 2021, 97, 328–348.
- Ferrando, S.J.; Klepacz, L.; Lynch, S.; Tavakkoli, M.; Dornbush, R.; Baharani, R.; Smolin, Y.; Bartell, A. COVID-19 Psychosis: A Potential New Neuropsychiatric Condition Triggered by Novel Coronavirus Infection and the Inflammatory Response? Psychosomatics 2020, 61, 551–555.
- Chaudhary, A.M.D.; Musavi, N.B.; Saboor, S.; Javed, S.; Khan, S.; Naveed, S. Psychosis during the COVID-19 pandemic: A systematic review of case reports and case series. J. Psychiatr. Res. 2022, 153, 37–55.
- Smith, C.M.; Gilbert, E.B.; Riordan, P.A.; Helmke, N.; von Isenburg, M.; Kincaid, B.R.; Shirey, K.G. Covid-19-associated psychosis: A systematic review of case reports. Gen. Hosp. Psychiatry 2021, 73, 84–100.
- O’Hanlon, S.; Inouye, S.K. Delirium: A missing piece in the COVID-19 pandemic puzzle. Age Ageing 2020, 49, 497–498.
- Otani, K.; Fukushima, H.; Matsuishi, K. COVID-19 delirium and encephalopathy: Pathophysiology assumed in the first 3 years of the ongoing pandemic. Brain Disord. 2023, 10, 100074.
- Rebora, P.; Rozzini, R.; Bianchetti, A.; Blangiardo, P.; Marchegiani, A.; Piazzoli, A.; Mazzeo, F.; Cesaroni, G.; Chizzoli, A.; Guerini, F.; et al. Delirium in patients with SARS-CoV-2 infection: A multicenter study. J. Am. Geriatr. Soc. 2020, 69, 293–299.
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162.
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232.
- Efstathiou, V.; Stefanou, M.-I.; Demetriou, M.; Siafakas, N.; Makris, M.; Tsivgoulis, G.; Zoumpourlis, V.; Kympouropoulos, S.; Tsoporis, J.; Spandidos, D.; et al. Long Covid and neuropsychiatric manifestations (review). Exp. Ther. Med. 2022, 23, 363.
- Scarpelli, S.; Nadorff, M.R.; Bjorvatn, B.; Chung, F.; Dauvilliers, Y.; Espie, C.A.; Inoue, Y.; Matsui, K.; Merikanto, I.; Morin, C.M.; et al. Nightmares in People with COVID-19: Did Coronavirus Infect Our Dreams? Nat. Sci. Sleep 2022, 14, 93–108.
- Oaklander, A.L.; Mills, A.J.; Kelley, M.; Toran, L.S.; Smith, B.; Dalakas, M.C.; Nath, A. Peripheral Neuropathy Evaluations of Patients With Prolonged Long COVID. Neuroimmunol. Neuroinflamm. 2022, 9, e1146.
- Silva-Hernández, L.; Cabal-Paz, B.; Mayo-Canalejo, D.; Horga, A. Post-COVID symptoms of potential peripheral nervous and muscular origin. Neurol. Perspect. 2021, 1, S25–S30.
- Munblit, D.; Bobkova, P.; Spiridonova, E.; Shikhaleva, A.; Gamirova, A.; Blyuss, O.; Nekliudov, N.; Bugaeva, P.; Andreeva, M.; DunnGalvin, A.; et al. Sechenov StopCOVID Research Team Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin. Exp. Allergy 2021, 51, 1107–1120.
- Wahlgren, C.; Forsberg, G.; Divanoglou, A.; Östholm Balkhed, Å.; Niward, K.; Berg, S.; Levi, R. Two-year follow-up of patients with post-COVID-19 condition in Sweden: A prospective cohort study. Lancet Reg. Health Eur. 2023.
- Pilotto, A.; Cristillo, V.; Cotti Piccinelli, S.; Zoppi, N.; Bonzi, G.; Sattin, D.; Schiavolin, S.; Raggi, A.; Canale, A.; Gipponi, S.; et al. Long-term neurological manifestations of COVID-19: Prevalence and predictive factors. Neurol. Sci. 2021, 42, 4903–4907.
- Di Stefano, G.; Falco, P.; Galosi, E.; Di Pietro, G.; Leone, C.; Truini, A. A systematic review and metanalysis of neuropathic pain associated with coronavirus disease 2019. Eur. J. Pain 2023, 27, 44–53.
- Fernández-de-Las-Peñas, C.; Nijs, J.; Neblett, R.; Polli, A.; Moens, M.; Goudman, L.; Shekhar Patil, M.; Knaggs, R.D.; Pickering, G.; Arendt-Nielsen, L. Phenotyping Post-COVID Pain as a Nociceptive, Neuropathic, or Nociplastic Pain Condition. Biomedicines 2022, 10, 2562.
- Burakgazi, A.Z. Small-Fiber Neuropathy Possibly Associated with COVID-19. Case Rep. Neurol. 2022, 14, 208–212.
- Odozor, C.U.; Kannampallil, T.; Ben Abdallah, A.; Roles, K.; Burk, C.; Warner, B.C.; Alaverdyan, H.; Clifford, D.B.; Piccirillo, J.F.; Haroutounian, S. Post-acute sensory neurological sequelae in patients with severe acute respiratory syndrome coronavirus 2 infection: The COVID-PN observational cohort study. Pain 2022, 163, 2398–2410.
- Grieco, T.; Gomes, V.; Rossi, A.; Cantisani, C.; Greco, M.E.; Rossi, G.; Sernicola, A.; Pellacani, G. The Pathological Culprit of Neuropathic Skin Pain in Long COVID-19 Patients: A Case Series. J. Clin. Med. 2022, 11, 4474.
- Hinduja, A.; Moutairou, A.; Calvet, J.H. Sudomotor dysfunction in patients recovered from COVID-19. Neurophysiol. Clin. 2021, 51, 193–196.
- Zis, P.; Ioannou, C.; Artemiadis, A.; Christodoulou, K.; Kalampokini, S.; Hadjigeorgiou, G.M. Prevalence and Determinants of Chronic Pain Post-COVID: Cross-Sectional Study. J. Clin. Med. 2022, 11, 5569.
- Abrams, R.M.C.; Simpson, D.M.; Navis, A.; Jette, N.; Zhou, L.; Shin, S.C. Small fiber neuropathy associated with SARS-CoV-2 infection. Muscle Nerve 2022, 65, 440–443.
- Pinzon, R.T.; Wijaya, V.O.; Jody, A.A.; Nunsio, P.N.; Buana, R.B. Persistent neurological manifestations in long COVID-19 syndrome: A systematic review and meta-analysis. J. Infect. Public Health 2022, 15, 856–869.
- Irisson-Mora, I.; Salgado-Cordero, A.M.; Reyes-Varón, E.; Cataneo-Piña, D.J.; Fernández-Sánchez, M.; Buendía-Roldán, I.; Salazar-Lezama, M.A.; Occupational Health and Preventive Medicine Consortium. Comparison between the persistence of post COVID-19 symptoms on critical patients requiring invasive mechanical ventilation and non-critical patients. PLoS ONE 2022, 17, e0273041.
- Ser, M.H.; Çalıkuşu, F.Z.; Tanrıverdi, U.; Abbaszade, H.; Hakyemez, S.; Balkan, İ.İ.; Karaali, R.; Gündüz, A. Autonomic and neuropathic complaints of long-COVID objectified: An investigation from electrophysiological perspective. Neurol. Sci. 2022, 43, 6167–6177.
- Needham, E.; Newcombe, V.; Michell, A.; Thornton, R.; Grainger, A.; Anwar, F.; Warburton, E.; Menon, D.; Trivedi, M.; Sawcer, S. Mononeuritis multiplex: An unexpectedly common feature of severe COVID-19. J. Neurol. 2021, 268, 2685–2689.
- Mahmood, S.B.Z.; Mushtaq, M.Z.; Kanwar, D.; Ali, S.A. Lower limb axonal mononeuropathies as sequelae of COVID-19: A case report and review of literature. Egypt. J. Neurol. Psychiatr. Neurosurg. 2022, 58, 22.
- Carberry, N.; Badu, H.; Ulane, C.M.; Beckley, A.; Rosenberg, S.J.; Brenner, K.; Brannagan, T.H. Mononeuropathy Multiplex After COVID-19. J. Clin. Neuromuscul. Dis. 2021, 23, 24–30.
- Law, S.M.; Scott, K.; Alkarn, A.; Mahjoub, A.; Mallik, A.K.; Roditi, G.; Choo-Kang, B. COVID-19 associated phrenic nerve mononeuritis: A case series. Thorax 2022, 77, 834–838.
- Palaiodimou, L.; Stefanou, M.I.; Katsanos, A.H.; Fragkou, P.C.; Papadopoulou, M.; Moschovos, C.; Michopoulos, I.; Kokotis, P.; Bakirtzis, C.; Naska, A.; et al. Prevalence, clinical characteristics and outcomes of Guillain-Barré syndrome spectrum associated with COVID-19: A systematic review and meta-analysis. Eur. J. Neurol. 2021, 28, 3517–3529.
- Yaqoob, A.; Dar, W.; Khuja, Z.; Bukhari, I.; Raina, A.; Ganie, H.; Chandra, A.; Wani, M.; Asimi, R.; Wani, F. Miller Fisher syndrome associated with COVID 19. J. Family Med. Prim. Care 2022, 11, 4023–4025.
- Malekpour, M.; Khanmohammadi, S.; Meybodi, M.J.E.; Shekouh, D.; Rahmanian, M.R.; Kardeh, S.; Azarpira, N. COVID-19 as a trigger of Guillain-Barré syndrome: A review of the molecular mechanism. Immun. Inflamm. Dis. 2023, 11, e875.
- Noon, A.; Malhi, J.K.; Wong, C.K. Atypical Guillain-Barré Syndrome Presenting After COVID-19 Infection. Cureus 2022, 14, e29521.
- Finsterer, J.; Scorza, F.A.; Scorza, C.A.; Fiorini, A.C. Peripheral neuropathy in COVID-19 is due to immune-mechanisms, pre-existing risk factors, anti-viral drugs, or bedding in the Intensive Care Unit. Arq. Neuropsiquiatr. 2021, 79, 924–928.
- Liu, X.; Treister, R.; Lang, M.; Oaklander, A.L. IVIg for apparently autoimmune small-fiber polyneuropathy: First analysis of efficacy and safety. Ther. Adv. Neurol. Disord. 2018, 11, 1756285617744484.
- Franke, C.; Berlit, P.; Prüss, H. Neurological manifestations of post-COVID-19 syndrome S1-guideline of the German society of neurology. Neurol. Res. Pract. 2022, 4, 28.
- Utrero-Rico, A.; Ruiz-Ruigómez, M.; Laguna-Goya, R.; Arrieta-Ortubay, E.; Chivite-Lacaba, M.; González-Cuadrado, C.; Lalueza, A.; Almendro-Vazquez, P.; Serrano, A.; Aguado, J.M.; et al. A Short Corticosteroid Course Reduces Symptoms and Immunological Alterations Underlying Long-COVID. Biomedicines 2021, 9, 1540.
- McWilliam, M.; Samuel, M.; Alkufri, F.H. Neuropathic pain post-COVID-19: A case report. BMJ Case Rep. 2021, 14, e243459.
- Thompson, J.S.; Thornton, A.C.; Ainger, T.; Garvy, B.A. Long-term high-dose immunoglobulin successfully treats Long COVID patients with pulmonary, neurologic, and cardiologic symptoms. Front. Immunol. 2023, 13, 1033651.
- Attal, N.; Martinez, V.; Bouhassira, D. Potential for increased prevalence of neuropathic pain after the COVID-19 pandemic. Pain Rep. 2021, 6, e884.
- El-Tallawy, S.N.; Perglozzi, J.V.; Ahmed, R.S.; Kaki, A.M.; Nagiub, M.S.; LeQuang, J.K.; Hadarah, M.M. Pain Management in the Post-COVID Era-An Update: A Narrative Review. Pain Ther. 2023, 12, 423–448.
- Córdova-Martínez, A.; Caballero-García, A.; Pérez-Valdecantos, D.; Roche, E.; Noriega-González, D.C. Peripheral Neuropathies Derived from COVID-19: New Perspectives for Treatment. Biomedicines 2022, 10, 1051.
- Figueroa-Padilla, I.; Rivera Fernández, D.E.; Cházaro Rocha, E.F.; Eugenio Gutiérrez, A.L.; Jáuregui-Renaud, K. Body Weight May Have a Role on Neuropathy and Mobility after Moderate to Severe COVID-19: An Exploratory Study. Medicina 2022, 58, 1401.
- Fernández-de-Las-Peñas, C.; Navarro-Santana, M.; Plaza-Manzano, G.; Palacios-Ceña, D.; Arendt-Nielsen, L. Time course prevalence of post-COVID pain symptoms of musculoskeletal origin in patients who had survived severe acute respiratory syndrome coronavirus 2 infection: A systematic review and meta-analysis. Pain 2022, 163, 1220–1231.
- Karaarslan, F.; Güneri, F.D.; Kardeş, S. Long COVID: Rheumatologic/musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months. Clin. Rheumatol. 2022, 41, 289–296.
- Maamar, M.; Artime, A.; Pariente, E.; Fierro, P.; Ruiz, Y.; Gutiérrez, S.; Tobalina, M.; Díaz-Salazar, S.; Ramos, C.; Olmos, J.M.; et al. Post-COVID-19 syndrome, low-grade inflammation and inflammatory markers: A cross-sectional study. Curr. Med. Res. Opin. 2022, 38, 901–909.
- Shanthanna, H.; Nelson, A.M.; Kissoon, N.; Narouze, S. The COVID-19 pandemic and its consequences for chronic pain: A narrative review. Anaesthesia 2022, 77, 1039–1050.
- Azadvari, M.; Haghparast, A.; Nakhostin-Ansari, A.; Emami Razavi, S.Z.; Hosseini, M. Musculoskeletal symptoms in patients with long COVID: A cross-sectional study on Iranian patients. Heliyon 2022, 8, e10148.
- Galluzzo, V.; Zazzara, M.B.; Ciciarello, F.; Tosato, M.; Martone, A.M.; Pais, C.; Savera, G.; Calvani, R.; Picca, A.; Marzetti, E.; et al. Inadequate Physical Activity Is Associated with Worse Physical Function in a Sample of COVID-19 Survivors with Post-Acute Symptoms. J. Clin. Med. 2023, 12, 2517.
- Guerrero, J.I.; Barragán, L.A.; Martínez, J.D.; Montoya, J.P.; Peña, A.; Sobrino, F.E.; Tovar-Spinoza, Z.; Ghotme, K.A. Central and peripheral nervous system involvement by COVID-19: A systematic review of the pathophysiology, clinical manifestations, neuropathology, neuroimaging, electrophysiology, and cerebrospinal fluid findings. BMC Infect. Dis. 2021, 21, 515.
- Dayaramani, C.; De Leon, J.; Reiss, A.B. Cardiovascular Disease Complicating COVID-19 in the Elderly. Medicina 2021, 57, 833.
- Moghimi, N.; Di Napoli, M.; Biller, J.; Siegler, J.E.; Shekhar, R.; McCullough, L.D.; Harkins, M.S.; Hong, E.; Alaouieh, D.A.; Mansueto, G.; et al. The Neurological Manifestations of Post-Acute Sequelae of SARS-CoV-2 infection. Curr. Neurol. Neurosci. Rep. 2021, 21, 44.
- Taga, A.; Lauria, G. COVID-19 and the peripheral nervous system. A 2-year review from the pandemic to the vaccine era. J. Peripher. Nerv. Syst. 2022, 27, 4–30.
- Michaelson, N.M.; Malhotra, A.; Wang, Z.; Heier, L.; Tanji, K.; Wolfe, S.; Gupta, A.; MacGowan, D. Peripheral neurological complications during COVID-19: A single center experience. J. Neurol. Sci. 2022, 434, 120118.
- Saif, A.; Pick, A. Polyneuropathy following COVID-19 infection: The rehabilitation approach. BMJ Case Rep. 2021, 14, e242330.
- Mahboubi Mehrabani, M.; Karvandi, M.S.; Maafi, P.; Doroudian, M. Neurological complications associated with Covid-19; molecular mechanisms and therapeutic approaches. Rev. Med. Virol. 2022, 32, e2334.
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144.
- Hartung, T.J.; Neumann, C.; Bahmer, T.; Chaplinskaya-Sobol, I.; Endres, M.; Geritz, J.; Haeusler, K.G.; Heuschmann, P.U.; Hildesheim, H.; Hinz, A.; et al. Fatigue and cognitive impairment after COVID-19: A prospective multicentre study. EClinicalMedicine 2022, 53, 101651.
- Albu, S.; Zozaya, N.R.; Murillo, N.; García-Molina, A.; Chacón, C.A.F.; Kumru, H. Multidisciplinary outpatient rehabilitation of physical and neurological sequelae and persistent symptoms of covid-19: A prospective, observational cohort study. Disabil. Rehabil. 2022, 44, 6833–6840.
- Badenoch, J.B.; Rengasamy, E.R.; Watson, C.; Jansen, K.; Chakraborty, S.; Sundaram, R.D.; Hafeez, D.; Burchill, E.; Saini, A.; Thomas, L.; et al. Persistent neuropsychiatric symptoms after COVID-19: A systematic review and meta-analysis. Brain Commun. 2021, 4, fcab297.
- Delgado-Alonso, C.; Valles-Salgado, M.; Delgado-Álvarez, A.; Yus, M.; Gómez-Ruiz, N.; Jorquera, M.; Polidura, C.; Gil, M.J.; Marcos, A.; Matías-Guiu, J.; et al. Cognitive dysfunction associated with COVID-19: A comprehensive neuropsychological study. J. Psychiatr. Res. 2022, 150, 40–46.
- Santoyo-Mora, M.; Villaseñor-Mora, C.; Cardona-Torres, L.M.; Martínez-Nolasco, J.J.; Barranco-Gutiérrez, A.I.; Padilla-Medina, J.A.; Bravo-Sánchez, M.G. COVID-19 Long-Term Effects: Is There an Impact on the Simple Reaction Time and Alternative-Forced Choice on Recovered Patients? Brain Sci. 2022, 12, 1258.
- Llana, T.; Zorzo, C.; Mendez-Lopez, M.; Mendez, M. Memory alterations after COVID-19 infection: A systematic review. Appl. Neuropsychol. Adult 2022.
- Daugherty, S.E.; Guo, Y.; Heath, K.; Dasmariñas, M.C.; Jubilo, K.G.; Samranvedhya, J.; Lipsitch, M.; Cohen, K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ 2021, 373, n1098.
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085.
- Wild, C.J.; Norton, L.; Menon, D.K.; Ripsman, D.A.; Swartz, R.H.; Owen, A.M. Disentangling the cognitive, physical, and mental health sequelae of COVID-19. Cell Rep. Med. 2022, 3, 100750.
- Jennings, G.; Monaghan, A.; Xue, F.; Duggan, E.; Romero-Ortuño, R. Comprehensive clinical characterisation of brain fog in adults reporting long covid symptoms. J. Clin. Med. 2022, 11, 3440.
- Asadi-Pooya, A.A.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Nemati, H.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; Farjoud-Kouhanjani, M.; et al. Long Covid syndrome-associated brain fog. J. Med. Virol. 2021, 94, 979–984.
- Taquet, M.; Sillett, R.; Zhu, L.; Mendel, J.; Camplisson, I.; Dercon, Q.; Harrison, P.J. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: An analysis of 2-year retrospective cohort studies including 1,284,437 patients. Lancet Psychiatry 2022, 9, 815–827.
- Moy, F.M.; Hairi, N.N.; Lim, E.; Bulgiba, A. Long COVID and its associated factors among COVID survivors in the community from a middle-income country-An online cross-sectional study. PLoS ONE 2022, 17, e0273364.
- Krishnan, K.; Lin, Y.F.; Prewitt, K.-R.M.; Potter, D.A. Multidisciplinary approach to brain fog and related persisting symptoms post covid-19. J. Health Serv. Psychol. 2022, 48, 31–38.
- Whiteside, D.M.; Basso, M.R.; Naini, S.M.; Porter, J.; Holker, E.; Waldron, E.J.; Melnik, T.E.; Niskanen, N.; Taylor, S.E. Outcomes in post-acute sequelae of COVID-19 (PASC) at 6 months post-infection Part 1: Cognitive functioning. Clin. Neuropsychol. 2022, 36, 806–828.
- Cristillo, V.; Pilotto, A.; Piccinelli, S.C.; Gipponi, S.; Leonardi, M.; Bezzi, M.; Padovani, A. Predictors of “brain fog” 1 year after COVID-19 disease. Neurol. Sci. 2022, 43, 5795–5797.
- Taube, M. Depression and brain fog as long-COVID mental health consequences: Difficult, complex and partially successful treatment of a 72-year-old patient-A case report. Front. Psychiatry 2023, 14, 1153512.
- Hugon, J. Long-COVID: Cognitive deficits (brain fog) and brain lesions in non-hospitalized patients. Presse Med. 2022, 51, 104090.
- Kao, J.; Frankland, P.W. Covid Fog Demystified. Cell 2022, 185, 2391–2393.
- Nuber-Champier, A.; Cionca, A.; Breville, G.; Voruz, P.; de Alcântara, I.J.; Allali, G.; Lalive, P.H.; Benzakour, L.; Lövblad, K.O.; Braillard, O.; et al. Acute TNFα levels predict cognitive impairment 6-9 months after COVID-19 infection. Psychoneuroendocrinology 2023, 153, 106104.
- He, D.; Yuan, M.; Dang, W.; Bai, L.; Yang, R.; Wang, J.; Ma, Y.; Liu, B.; Liu, S.; Zhang, S.; et al. Long term neuropsychiatric consequences in COVID-19 survivors: Cognitive impairment and inflammatory underpinnings fifteen months after discharge. Asian J. Psychiatr. 2023, 80, 103409.
- Jeong, G.U.; Lyu, J.; Kim, K.D.; Chung, Y.C.; Yoon, G.Y.; Lee, S.; Hwang, I.; Shin, W.H.; Ko, J.; Lee, J.Y.; et al. SARS-CoV-2 infection of microglia elicits proinflammatory activation and apoptotic cell death. Microbiol. Spectr. 2022, 10, e0109122.
- Marshall, M. COVID and the brain: Researchers zero in on how damage occurs. Nature 2021, 595, 484–485.
- McMahon, C.L.; Staples, H.; Gazi, M.; Carrion, R.; Hsieh, J. SARS-CoV-2 targets glial cells in human cortical organoids. Stem Cell Rep. 2021, 16, 1156–1164.
- Goldstein Ferber, S.; Shoval, G.; Zalsman, G.; Weller, A. Does COVID-19 related symptomatology indicate a transdiagnostic neuropsychiatric disorder?—Multidisciplinary implications. World J. Psychiatry 2022, 12, 1004–1015.
- Trecca, E.M.C.; Cassano, M.; Longo, F.; Petrone, P.; Miani, C.; Hummel, T.; Gelardi, M. Results from psychophysical tests of smell and taste during the course of SARS-CoV-2 infection: A review. Acta Otorhinolaryngol. Ital. 2022, 42, S20–S35.
- Costa Dos Santos, J.; Ximenes Rabelo, M.; Mattana Sebben, L.; de Souza Carneiro, M.V.; Bosco Lopes Botelho, J.; Cardoso Neto, J.; Nogueira Barbosa, A.; Monteiro de Carvalho, D.; Pontes, G.S. Persistence of SARS-CoV-2 Antigens in the Nasal Mucosa of Eight Patients with Inflammatory Rhinopathy for over 80 Days following Mild COVID-19 Diagnosis. Viruses 2023, 15, 899.
- Tsuchiya, H. Oral Symptoms Associated with COVID-19 and Their Pathogenic Mechanisms: A Literature Review. Dent. J. 2021, 9, 32.
- Han, A.Y.; Mukdad, L.; Long, J.L.; Lopez, I.A. Anosmia in COVID-19: Mechanisms and significance. Chem. Senses 2020, 45, 423–428.
- Trott, M.; Driscoll, R.; Pardhan, S. The prevalence of sensory changes in post-COVID syndrome: A systematic review and meta-analysis. Front. Med. 2022, 9, 980253.
- Chudzik, M.; Babicki, M.; Mastalerz-Migas, A.; Kapusta, J. Persisting Smell and Taste Disorders in Patients Who Recovered from SARS-CoV-2 Virus Infection-Data from the Polish PoLoCOV-CVD Study. Viruses 2022, 14, 1763.
- Tan, B.K.J.; Han, R.; Zhao, J.J.; Tan, N.K.W.; Quah, E.S.H.; Tan, C.J.; Chan, Y.H.; Teo, N.W.Y.; Charn, T.C.; See, A.; et al. Prognosis and persistence of smell and taste dysfunction in patients with covid-19: Meta-analysis with parametric cure modelling of recovery curves. BMJ 2022, 378, e069503.
- Helmsdal, G.; Hanusson, K.D.; Kristiansen, M.F.; Foldbo, B.M.; Danielsen, M.E.; Steig, B.Á.; Gaini, S.; Strøm, M.; Weihe, P.; Petersen, M.S. Long COVID in the Long Run-23-Month Follow-up Study of Persistent Symptoms. Open Forum Infect. Dis. 2022, 9, ofac270.
- Prem, B.; Liu, D.T.; Besser, G.; Sharma, G.; Dultinger, L.E.; Hofer, S.V.; Matiasczyk, M.M.; Renner, B.; Mueller, C.A. Long-lasting olfactory dysfunction in COVID-19 patients. Eur. Arch. Otorhinolaryngol. 2021, 279, 3485–3492.
- Boscolo-Rizzo, P.; Tofanelli, M.; Zanelli, E.; Gardenal, N.; Tirelli, G. COVID-19-Related Quantitative and Qualitative Olfactory and Gustatory Dysfunction: Long-Term Prevalence and Recovery Rate. ORL J. Otorhinolaryngol. Relat. Spec. 2023, 85, 67–71.
- Rashid, R.A.; Alaqeedy, A.A.; Al-Ani, R.M. Parosmia Due to COVID-19 Disease: A 268 Case Series. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 2970–2977.
- Melley, L.E.; Bress, E.; Polan, E. Hypogeusia as the initial presenting symptom of COVID-19. BMJ Case Rep. 2020, 13, e236080.
- Tuter, G.; Yerebakan, M.; Celik, B.; Kara, G. Oral manifestations in SARS-CoV-2 infection. Med. Oral Patol. Oral Cir. Bucal. 2022, 27, e330–e339.
- Park, G.C.; Bang, S.Y.; Lee, H.W.; Choi, K.U.; Kim, J.M.; Shin, S.C.; Cheon, Y.I.; Sung, E.S.; Lee, M.; Lee, J.C.; et al. ACE2 and TMPRSS2 immunolocalization and oral manifestations of COVID-19. Oral Dis. 2022, 28, 2456–2464.
- Vandersteen, C.; Payne, M.; Dumas, L.É.; Cancian, É.; Plonka, A.; D’Andréa, G.; Chirio, D.; Demonchy, É.; Risso, K.; Askenazy-Gittard, F.; et al. Olfactory Training in Post-COVID-19 Persistent Olfactory Disorders: Value Normalization for Threshold but Not Identification. J. Clin. Med. 2022, 11, 3275.
- Donelli, D.; Antonelli, M.; Valussi, M. Olfactory training with essential oils for patients with post-COVID-19 smell dysfunction: A case series. Eur. J. Integr. Med. 2023, 60, 102253.
- Ludwig, S.; Schell, A.; Berkemann, M.; Jungbauer, F.; Zaubitzer, L.; Huber, L.; Warken, C.; Held, V.; Kusnik, A.; Teufel, A.; et al. Post-COVID-19 Impairment of the Senses of Smell, Taste, Hearing, and Balance. Viruses 2022, 14, 849.
- Degen, C.V.; Mikuteit, M.; Niewolik, J.; Joosten, T.; Schröder, D.; Vahldiek, K.; Mücke, U.; Heinemann, S.; Müller, F.; Behrens, G.M.N.; et al. Audiological profile of adult Long COVID patients. Am. J. Otolaryngol. 2022, 43, 103579.
- De Luca, P.; Di Stadio, A.; Colacurcio, V.; Marra, P.; Scarpa, A.; Ricciardiello, F.; Cassandro, C.; Camaioni, A.; Cassandro, E. COVID, audiovestibular symptoms and persistent chemosensory dysfunction: A systematic review of the current evidence. Acta Otorhinolaryngol. Ital. 2022, 42, S87–S93.
- McFadyen, J.D.; Stevens, H.; Peter, K. The emerging threat of (micro)thrombosis in COVID-19 and Its therapeutic implications. Circ. Res. 2020, 127, 571–587.
- Dorobisz, K.; Pazdro-Zastawny, K.; Misiak, P.; Kruk-Krzemień, A.; Zatoński, T. Sensorineural Hearing Loss in Patients with Long-COVID-19: Objective and Behavioral Audiometric Findings. Infect. Drug Resist. 2023, 16, 1931–1939.
- Coelho, D.H.; Reiter, E.R.; French, E.; Costanzo, R.M. Decreasing Incidence of Chemosensory Changes by COVID-19 Variant. Otolaryngol. Head Neck Surg. 2023, 168, 704–706.
- Zazhytska, M.; Kodra, A.; Hoagland, D.A.; Frere, J.; Fullard, J.F.; Shayya, H.; McArthur, N.G.; Moeller, R.; Uhl, S.; Omer, A.D.; et al. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell 2022, 185, 1052–1064.
- Sanyaolu, A.; Marinkovic, A.; Prakash, S.; Zhao, A.; Balendra, V.; Haider, N.; Jain, I.; Simic, T.; Okorie, C. Post-acute Sequelae in COVID-19 Survivors: An Overview. SN Compr. Clin. Med. 2022, 4, 91.
- Öncül, H.; Öncül, F.Y.; Alakus, M.F.; Çağlayan, M.; Dag, U. Ocular findings in patients with coronavirus disease 2019 (COVID-19) in an outbreak hospital. J. Med. Virol. 2020, 93, 1126–1132.
- Tohamy, D.; Sharaf, M.; Abdelazeem, K.; Saleh, M.G.A.; Rateb, M.F.; Soliman, W.; Kedwany, S.M.; Omar Abdelmalek, M.; Medhat, M.A.; Tohamy, A.M.; et al. Ocular manifestations of post-acute covid-19 syndrome, Upper Egypt Early Report. J. Multidiscip. Healthc. 2021, 14, 1935–1944.
- Kazantzis, D.; Machairoudia, G.; Theodossiadis, G.; Theodossiadis, P.; Chatziralli, I. Retinal microvascular changes in patients recovered from COVID-19 compared to healthy controls: A meta-analysis. Photodiagnosis Photodyn. Ther. 2023, 42, 103556.
- Jevnikar, K.; Meglič, A.; Lapajne, L.; Logar, M.; Vidovič Valentinčič, N.; Globočnik Petrovič, M.; Jaki Mekjavić, P. The Comparison of Retinal Microvascular Findings in Acute COVID-19 and 1-Year after Hospital Discharge Assessed with Multimodal Imaging-A Prospective Longitudinal Cohort Study. Int. J. Mol. Sci. 2023, 24, 4032.
- Johansson, J.; Möller, M.; Markovic, G.; Borg, K. Vision impairment is common in non-hospitalised patients with post-COVID-19 syndrome. Clin. Exp. Optom. 2023; Advance online publication.
