1. Introduction
Enzymatic hydrolysis (EH) is the step of the bioconversion process during which fermentable sugar monomers are liberated from the biomasses’ structural carbohydrates, cellulose and hemicellulose [
155,
156]. Cellulases target cellulose and cleave β-1,4-D-glucan linkages in the polymer, while hemicellulases breakdown galactan, xylan, mannan, and araban, having similar activities to cellulases, because the same linkages can be found in the hemicellulose structure [
157,
158,
159]. One type of hemicellulase, known as endoxylanase, targets the β-d xylano pyranosyl bonds within xylan to release xylo-oligosaccharides [
160,
161]. Cellulose–glucose conversion generally takes place at temperatures of 40–50 °C and a pH of around 4.8, yet the efficiency of the process relies on other influential factors, namely lignin removal, solubilization of hemicellulose, acetylation of hemicellulose, hydrolysis duration, enzyme loading, cellulose crystallinity, the presence of surfactants, biomass particle size, pore volume, and accessible surface area [
159,
162,
163]. This confirms, once again that PT operating conditions have a considerable impact on the subsequent process. Mussatto et al. [
164] have evaluated the influence of enzyme loading, substrate concentration, and agitation speed on the outcomes of EH. While agitation speed had the lowest impact, the results showed that enzyme loading had the highest impact. Solid loading also plays an important role in the efficiency of EH; loadings less than 6–10%
w/
v make water more available, thus decreasing the reaction–diffusion limitations. The only drawback of low-solids EH is the increased production cost and energy demand [
165,
166]. Increasing substrate loading has its own disadvantages too, which include increased viscosity and the restraint of heat-mass transfers [
167].
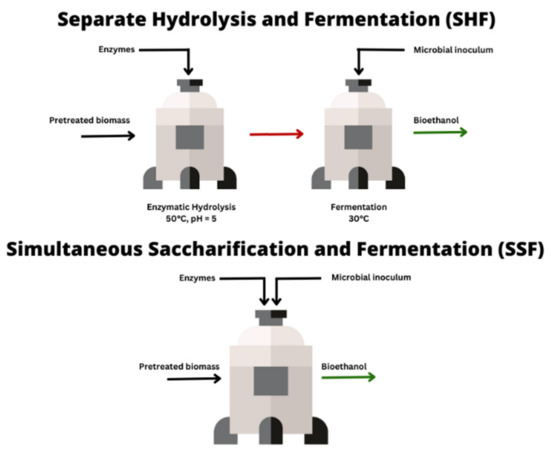
When the bioconversion process comprises both EH and fermentation, the former can either be conducted separately from the latter step (separate hydrolysis and fermentation; SHF) or together in one step (simultaneous saccharification and fermentation; SSF). Generally, studies have reported that SSF is more efficient than EH followed by fermentation, because it limits inhibitions that can be observed during SHF and it helps to reduce costs [
168,
169,
170] (
Figure 1).
Figure 1. Comparing SHF and SSF.
When it comes to fermentation,
Saccharomyces cerevisiae is the most commonly used microorganism for the conversion of glucose into ethanol. However, a limitation of
S. cerevisiae is its inability to ferment pentoses, which are released during the EH of hemicellulose [
171]. This challenge has prompted the development of genetically engineered strains capable of metabolizing these sugars, thereby enabling more efficient bioconversion processes.
2. Enzymatic Hydrolysis of Woody Biomass after Thermomechanical PT
Thermo-mechanically treated eucalyptus sawdust [
59,
88,
90], poplar wood [
91], spruce wood [
95],
Aucoumea wood [
93], hornbeam wood [
92], aspen wood [
94], and hemp hurds [
26] were subjected to enzymatic hydrolysis. The severity of the PT conditions had various effects on EH across different studies. For instance, when NaOH-impregnated and non-impregnated eucalyptus sawdust [
59] were hydrolyzed, the results showed that the enzymatic digestibility of the non-impregnated solids attained an efficiency of 96% at 200 °C after optimization, producing 134 g/L of glucose. Interestingly, in this particular case, the impregnation step with a catalyst solution did not promote enzymatic hydrolysis, which contradicts previous findings and warrants further investigation. Another example would be the results obtained in the works of Romaní et al. on SE-treated eucalyptus wood [
88], in which a conversion efficiency of 94.5% following PT optimization (195 °C, 5.87 min) was reached, and the resulting (xylose + oligosaccharides) concentration was about 18.1 g/100 g oven-dried wood. On the other hand, the PT of spruce wood [
95] allowed the liberation of both glucose and mannose, which increased with PTs even at lower severities, and without any degradation of pentoses. This indicates that sugar molecules are preserved at specific severities, allowing for their recovery through EH, whereas higher PT severities may induce their degradation. However, and as already discussed, Pielhop et al. concluded that a ΔP > 5 bar is required for enhanced enzymatic digestibility, which was confirmed by the highest total sugar yield obtained (62%) at a PT severity of 4.7 (without catalyst). Zhou et al. [
172] proved that increased PT severity influences the enzyme digestibility of corn stover, but not necessarily positively for all hydrolysis durations. They reported that their highest glucose yield (89.2%) was obtained at a severity of 3.716 (210 °C, 3 min), while just increasing the time from 3 to 10 min (severity of 4.239) led to a degradation of cellulose, thus slightly lowering the glucose yield. While the dependency of sugar yield and inhibitor production on temperature was observed, the correlation with PT severity was determined by the nature or the composition of the biomasses. Generally exhibiting a lower recalcitrance than woods, corn stover and other herbaceous biomasses are more sensitive to PT conditions, and have a higher risk of sugar degradation. Nitsos et al. [
173] pretreated poplar residues and pine sawdust chemically, and tested several PT severities. Their results revealed low concentrations of major byproducts for the whole series of severities (R
0 = 3.8–4.1), which further confirms the influence of the biomass’s nature. In contrast, Besserer et al. [
93] concluded that lower severities (170–190 °C, 2.5–5 min, 0.25–0.5% H
2SO
4) resulted in low glucose yields, while an increase to 210 °C for 5 min with the addition of 0.25% H
2SO
4 resulted in 23.4 g glucose/100 g DW of wood. Notably, the washing step practiced by Besserer et al. provided an advantage by reducing the inhibitors, which consequently impacted the EH outcomes. In contrast, Mihiretu et al. [
94] achieved their highest glucose yield at 204 °C over 10 min (approximately 82 g/L of glucose), with alkaline impregnation (5%
w/
w NaOH). Both furfural and HMF were detectable at higher severities (200 °C, 15 min, 5%
w/
w NaOH), but were significantly higher at lower severity (190 °C, 10 min, no alkali agent). Further investigation on the correlation between sugar yield, inhibitor formation, and PT severity is mandatory.
On another note, the works of Pažitný et al. [
91] have highlighted that SE-treated poplar heartwood yielded the highest concentrations of both glucose and xylose (90 g/L after 72 h), and of glucose alone (70.4 g/L at 48 h). Studies performed on both larch wood [
174] and pine wood [
175] have both stated that sapwood possesses a higher cellulose content than heartwood, whereas heartwood has a higher lignin content. A similar distribution was also underlined in poplar tree parts [
91]. While one might anticipate that the elevated lignin content in poplar heartwood would lead to decreased glucose yields, it is possible that the lower lignin content in sapwood potentially facilitated cellulose degradation. This is evidenced by the maximum glucose yield obtained from poplar sapwood, which reached 65 g/L. Finally, the study of Semhaoui [
26] showed that the use of 0.66% H
2SO
4 significantly influenced the production of glucose and xylose. With 2% H
2SO
4, the maximum glucose and xylose concentrations obtained from 10 g of thermomechanically treated hemp hurds were 6.36 g/L and 2.1 g/L, respectively. The results of this study highlighted that non-catalyzed thermomechanical treatment (IV-HMT, 165 °C, 30 min) does not favor inhibitor production, the detected concentrations of which were negligible. The use of an acidic catalyst, as well as the increase in acid loading, led to greater formation of inhibitors. The project of Semhaoui [
90] has also emphasized that the use of an H
2SO
4 catalyst as an impregnation solution resulted in an overall greater reduction in sugar concentrations than using NaOH. At optimal PT conditions, 0.8% H
2SO
4 enabled a 90% yield in reducing sugars versus a reduction of 83.2% for alkaline-impregnated hemp hurds. These results suggest that NaOH not only solubilized lignin and hemicellulose, but also induced the formation of other particles that hindered EH. Furthermore, a reduction in specific surface area was noted for NaOH-impregnated hemp hurds. This phenomenon can be attributed to the likelihood of lignin condensation occurring in the alkaline environment at elevated temperatures, thereby obstructing access to open pores.
Last but not least, another attempt at process optimization was performed by Schneider et al. [
90]. After SE of eucalyptus wood, a biological post-PT using manufactured laccases from a strain of the saprophytic fungus
Marasmiellus palmivorus [
176] was conducted. The biological treatment aimed to detoxify the treated wood from the inhibitors produced, and was able to decrease the free phenolic compounds content found in soluble fraction by 70%. However, ethanol yield was approximately 10% greater when laccase treatment was performed after EH rather than after PT. Pretreated biomass detoxification has already been suggested as a promising additional step for an enhanced process; both biological and chemical PTs can be performed on LCB hydrolysates and slurries [
177,
178,
179].
3. Enzymatic Hydrolysis of Woody Biomass after Chemical PT
Chemically pretreated pine and poplar wood [
53], sawdust mixture [
126], hemp [
126], spruce [
133], olive wastes [
131], and paper mulberry wood [
115] were subjected to an enzymatic hydrolysis step separate from the fermentation phase, while chemically pretreated poplar wood, pine wood [
50,
68], and furniture boards [
114] were subjected to SSF. In the study led by Bay et al. [
53], the structural analyses confirmed that amorphous parts are broken down by enzymes before the crystalline parts [
180,
181,
182]. This is due to the fact that glycosidic bonds exist within the crystalline fraction and are less reactive to hydrolysis [
183]. The results also revealed that the PT of wood samples with H
3PO
4 and cold NaOH had the lowest α (1510/900) values (i.e., the lowest lignin-to-cellulose ratios), which indicates the greatest delignification. It can also be concluded that among all five PTs, H
3PO
4 and cold NaOH PTs allowed for the greatest glucose production from pretreated poplar and pine (493.3 g/kg substrate (H
3PO
4) and 459.2 g/kg substrate (cold NaOH) vs. 446 g/kg substrate (H
3PO
4) and 340.5 g/kg substrate (cold NaOH), respectively). Similarly, PHP PT of furniture boards [
114] further draws attention to the efficiency of H
3PO
4 in the enhancement of the bioconversion process, as 208–241 g glucose/kg of substrate was liberated from the hydrolysis of fiberboard, chipboard, and blockboard, using a high enzymatic loading of 20 mg protein/g cellulose. The experiments led by Zhao et al. [
114] also revealed that PHP PT decreased both crystal size and crystallinity index (CrI), through the dissolution and swelling of cellulose, which is originally crystalline [
184]. This reduction in crystallinity has been proven to increase surface area, thus enhancing enzymatic hydrolysis [
185,
186]. Furthermore, the effect of PHP PT on the saccharification of woody LCB was showcased in the experiments of Wan et al. [
68], in which the cellulose-to-glucose conversion was 40.4% for poplar and 27.5% for pine within the first 4 h. Wan et al. [
68] found that an almost complete conversion could be achieved within 48 h, while Zhao et al. [
181] reported that a 72 h incubation was necessary to achieve the maximum conversion yield. This could be explained by the difference in composition of the furniture boards compared to poplar and pine, as well as by the crystallinity post-PT of the substrates. Although PHP treatment reduces the CrI, some crystallinity could remain in the pretreated substrates, which consequently affect the rate of hydrolysis.
Alternatively, the preliminary hydrolysis tests of an organosolv-treated sawdust mixture [
126] revealed the influence of the substrate’s particle size on the overall process. Oven-dried particles greater than 0.5 mm produced less glucose (1.6 g/L) and had a slower cellulose-to-glucose conversion rate (12.4% within 8 days), as opposed to larger particles, which produced 3.1 g/L of glucose and had a conversion rate of 24%. Zhao et al. [
181] concluded that smaller particle sizes are expected to enhance enzyme digestibility, but this is not the case for particles smaller than 350–590 µm, with which no further significant enhancement was observed.
As previously mentioned, EH is influenced by multiple factors. Alio et al. [
126] tackled other influential parameters, which were solid and enzyme loading. The results demonstrated that under the chosen experimental conditions, an increase in enzyme loading (from 50 FPU/g to 70 FPU/g) for the same solid loading of 1.5%
m/
v improved the digestibility, as did an increase in solid loading (from 1.5%
m/
v to 5.2%
m/
v) for a fixed enzyme loading of 50 FPU/g. Moreover, a particularly high glucose yield of 443 mg glucose/g of pretreated wood was obtained in the study of Ajayo et al. [
115] after RSM optimization (H
3PO
4 71.3%
w/
w + H
2O2 4.84%
w/
w, 34.7 °C, 3.3 h). The study underlines that 100% hydrolysis could be achieved at a low H
3PO
4 fraction (60%) and high temperature (50 °C), while a higher acid fraction (80%) and lower-level temperature (30 °C) decreased hydrolysis efficiency to 69%. The study also showed the interaction between temperature and time, and its influence on hydrolysis yield, which reached 100% when both parameters were increased (41 °C, 2 h). Han et al. [
187] deduced that while prolonged time increased hydrolysis efficiently, PT durations exceeding 1.5 h led to a decrease in efficiency due to the possible degradation of the sugar polymers. It is worth mentioning that the PT duration was also influential in this case, as the NaOH PT was performed at 121 °C, with varying NaOH concentrations (0.25–1.5%
w/
v). From here, the consideration of PT severity remains critical for the preservation of the sugar polymers. Finally, the influence of surfactants on enzymatic hydrolysis was investigated in pretreated spruce wood [
188], and more recently in pretreated sugarcane bagasse, cypress sawdust, African coral wood [
189], and bamboo [
190]. These studies have tested the use of diverse surfactants, all of which had a positive effect on enzymatic hydrolysis. Nonetheless, since numerous previously used surfactants are not particularly environmentally friendly, the use of biosurfactants has been encouraged [
191,
192] as a more sustainable alternative.
4. Enzymatic Hydrolysis of Woody Biomass after Thermal/Thermochemical PT
Enzymatic hydrolysis was performed following the PT of aspen wood [
67], pine wood [
67,
146], beech chips [
146], teak wood [
148], hemp hurds [
145], sal sawdust [
149], and acacia wood [
151]. The double enzymatic hydrolysis performed by Sjulander et al. did not exhibit high efficiencies (overall glucose yield in aspen wood = 29.19% vs. 5.19% for pine wood) due to the reported repolymerization of lignin, as well as the possible inhibition of cellulases by mannan polysaccharides from pine wood. In contrast, the teak wood EH [
148] was followed by a detoxification step via two different methods: chemical, using Ca(OH)
2, and biological, using laccases (as observed in the study of Schneider et al. [
90]). After 36 h, the obtained glucose and xylose concentrations were 60 g/L and 20 g/L, respectively. While it was reported that the biological detoxification did not influence the fermentable sugars’ concentration, it did lead to a 40% decrease in soluble phenolic compounds, which act as inhibitors. Lime detoxification negatively influenced the sugar concentrations, reducing the glucose concentration by 7% and xylose concentration by 27%. This might be caused by the reaction between Ca(OH)
2 and the cleaved glucose molecules, as it produces the water-soluble molecule calcium glucosate [
193].
Mikulski et al. [
146] observed an increased susceptibility of two woody substrates to enzymatic hydrolysis following microwave-assisted PT using two solvents (NaCS and ethanol/H
2SO
4). The hydrolysis yield of pine chips following the ethanol/H
2SO
4 PT was 30.54%, as opposed to 56.84% for beech chips using the same solvent. However, the hydrolysis yield of beech chips was slightly greater using 1% NaOH (62.21%). Since cellulose and lignin contents were close in both NaCS-treated and NaOH-treated beech chips, the enhanced enzyme digestibility observed in the latter could be attributed to the lower extractive contents, which have been reported to have a positive effect on enzyme hydrolysis [
194].
The work of Dessie et al. [
145] took a completely different approach as part of their study. This approach consisted of a “one-pot PT and saccharification”, which finally achieved a maximal reducing sugar concentration of 39.49 g/L. The approach consisted of combining the thermochemically pretreated hemp hurds with mashes from the SSF step performed in the study; the chosen PT was OAA thermochemical PT using 2% oxalic acid. Surprisingly, not only did the greater acid concentration (3%) further reduce sugar concentrations, but it also increased the processing cost and formation of inhibitors. The one-pot PT and saccharification approach was also adopted by Shi et al. [
195], and more recently by Sriariyanun et al. [
196]. This “one-pot” approach would have been interesting with regard to time and space efficiency, as fewer specific units are required for each step of the bioconversion. However, if the pretreated substrate were not to exhibit successful delignification, or if microbial inhibitions were still present [
196], then perhaps the approach would not be of greater efficiency than a classic PT.
Finally, Lee et al. [
151] highlighted the efficiency of a mechanical refining post-PT of acacia wood, as well as the addition of soy protein, which increased the efficiency of the hydrolysis to 73.8%. The study explains how soy protein plays a role in enzymatic hydrolysis by preventing the irreversible binding of the enzyme to lignin [
197,
198].
This entry is adapted from the peer-reviewed paper 10.3390/en16135052