Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemical Research Methods
Cellulose is the most abundant natural polymer, which has attracted great attention due to the demand for eco-friendly and sustainable materials. The sustainability of cellulose products also depends on the selection of the dissolution solvent.
- cellulose
- ionic liquid
- hydrogel
- regeneration
1. Introduction
The development of products with durability, repairability, and reusability is a sustainable way to mitigate environmental impacts and move toward a circular economy. The impact on the environment has manifested from the continuous use of petroleum-based resources that must be substituted with alternative renewable and eco-friendly resources [1,2]. Biomass-based raw materials are a promising replacement of non-renewable, petroleum-based materials for advancing the efforts toward achieving a sustainable society. In order to achieve the desired levels of sustainable growth and to mitigate environmental pollution, biomass has been applied to reduce wastes and develop value-added products [3]. Expanding the use of such biomass-based materials does not only address the societal need for low-cost materials, but also reduces carbon footprints [4,5]. As a consequence of the abundance of renewable biomass materials in nature, its rational use is of great significance for the sustainable development of the chemical industry [6].
Cellulose is a biomass source with the potential to replace fossil fuels through its use in the production of value-added chemicals such as 5-hydroxymethylfurfural and levulinic acid [7]. It is a natural polymer available from diverse sources such as plants and from the extracellular production of several microbial genera, particularly Gluconacetobacter xylinus [8,9]. Cellulose can be obtained from wastes such as paper [10], textile [11,12,13], oil palm frond [14], and crop straw [15,16]. Due to its availability, biodegradability, non-toxicity, hydrophilic nature, inexpensive cost, and multifunctionality, cellulose is attractive and shows promise for utilization in sustainable material engineering. The processibility of cellulose allows it to be formed into various types of materials, for example, into hydrogels [17] and/or applied in its dry form as aerogels [18]. Cellulose hydrogels (jelly-like solids) are obtained by the regeneration or crosslinking of cellulose in solutions into a 3D shape, which are different from the non-cross-linked gel-like suspension of nano- or micro-fibrillated cellulose [19,20,21]. Due to the presence of hydroxyl groups in cellulose, the cross-linking in the hydrogel is formed through hydrogen bonding, which is reconstructed during the cellulose regeneration and solvent removal process [22]. Numerous hydroxyl groups in cellulose and other hydrophilic groups, e.g., carboxyl and amino groups, present in the cellulose derivatives, enable the entrapment of large volumes of water [23,24]. Depending on their applications in biotechnology, cosmetology, and ecology, a variety of sizes, from macro to nanogels, such as membranes, films, fibers, microspheres, or nanoparticles, can be fabricated by various interactions [25,26]. Those interactions are hydrogen bonding, electrostatic interactions, van der Waals forces, and physical entanglements of the cellulose molecules and/or their blends [25,26]. One of the main limitations of processing native cellulose is that it is difficult to dissolve in conventional organic or inorganic solvents due to the abundance of hydrogen bonds. The cellulose derivatives, e.g., carboxymethylcellulose, are much easier to dissolve, but multiple production steps are required to produce such derivatives [27]. The native cellulose is a semi-crystalline polymer comprising a high order of crystalline regions and a lower order of amorphous regions [28]. The rich hydrogen bonds between and within cellulose polymer chains are beneficial for the mechanical properties of cellulose crystals which greatly enhances mechanical strength of highly crystalline cellulose materials [29]. However, the resultant strong and complex 3D inter- and intramolecular hydrogen bonding network contributes to the chain rigidity and insolubility of cellulose, which must be disrupted for dissolution [30,31]. The bonding disruption can be achieved by the derivatization of hydroxyl groups of cellulose or comparative formation of a stronger hydrogen bond with other strong hydrogen-bonding solvents [30,31]. The solvents that have been used in the sol–gel process of cellulose are: N-dimethylacetamide (DMAc)/LiCl [32]; tetrabutylammonium fluoride/dimethyl sulfoxide (TBAF/DMSO) [33]; alkali/urea (NaOH/urea/H2O, LiOH/thiourea/H2O, etc.) [34,35]; N-methyl morpholine oxide (NMMO) [36,37]; NMMO/DMSO [38]; ZnCl2 aqueous solutions [39,40,41,42]; ZnCl2 molten salt hydrates [43]; molten hydrates of LiClO4 NaSCN/KSCN/LiSCN and LiCl/ZnCl2 [44]; molten salt hydrates of ZnBr2 and FeCl3 [45]; LiBr trihydrate molten salt [46]; and ethylenediamine solutions of NaSCN, KSCN, or NaI [44,47]. However, harsh dissolution conditions, such as high temperature, strong corrosiveness from the alkali metal hydroxide, and long processing time, have been reported [48]. Direct non-derivatizing dissolution of cellulose by the NMMO-based Lyocell process is commercialized. Although it is less-time consuming, the thermal stability and side reaction of NMMO, which decreases the performance of hydrogel fibers, remains a concern [49]. The ZnCl2 aqueous solutions and inorganic molten salt hydrates have been of interest as cellulose dissolution solvents in recent years due to their ease of preparation and low cost [41,42,50]. However, the solubility of cellulose in such inorganic salts is low and requires a high temperature or long dissolution time, causing energy consumption and hydrolysis [50,51].
Ionic liquids (ILs) are considered to be desirable eco-friendly solvents and have been used to replace the organic solvents for cellulose dissolution under milder conditions. A regular crystalline structure is difficult to form because of the asymmetry of ILs and the reduced electrostatic forces between the cations and anions [52]. Therefore, they can be liquid at low or room temperature. The ILs can form comparatively stronger hydrogen bonding with the native molecular hydrogen bond network within cellulose structures, causing hydrogen bonding disruption and dissolution. Due to their main advantages besides their high cellulose dissolution ability, ILs have low vapor pressure and are efficiently recycled in order to avoid their propagation in ecosystems. Cellulosic solutions in ILs can also be converted into ionogels or hydrogels by simple operations such as regeneration in cellulose antisolvents [53].
2. Ionic Liquids Used in Dissolution of Cellulose
2.1. Pure Ionic Liquid Systems
Anions and cations of ILs strongly affect cellulose solubility. The ILs containing cations with an unsaturated heterocyclic ring have the ability to dissolve cellulose because of their π electron delocalization of the ring. Such cations are active to interact with cellulose by providing more space for anions to form hydrogen bonds with cellulose. In contrast, the larger volume of ILs containing cations with the saturated heterocyclic ring slows down cations and anions transfer, which is less favorable for cellulose dissolution [54]. The options for cellulose dissolution have been ILs with aromatic imidazolium or pyridinium cations [55,56]. The imidazolium salts, such as 1-allyl-3-methyl imidazolium chloride (AmimCl), 1-buthyl-3-methyl imidazolium chloride (BmimCl), and 1-ethyl-3-methylimidazolium acetate (EmimAc), have received attention [57,58]. A series of substituted imidazolium cations, paired with lactate or glycolate anions, was developed by Meenatchi et al. [59]. For 5% w/w of cellulose solution, 1-ethyl imidazolium lactate showed the highest dissolution power within 20 min at 80 °C because of its higher number of acidic protons (C-2, C-4, C-5) which, in turn, favor the formation of more hydrogen bonds with oxygen atoms of cellulose. The replacement of the acidic C-2 proton by a methyl group (2-methylimidazolium) reduced the dissolution power, which suggested the substitution decreased the hydrogen bond interactions [59].
The anions also play a vital role in the breakage of the native cellulose hydrogen bond network because of the strong electronegativity, allowing anions to form strong hydrogen bonds with the hydroxide groups of cellulose. After being combined with various cations possessing alkyl functional groups, Cl−, acetate (Ac−), dimethyl phosphate (DMP−), and diethyl phosphate (DEP−) anions (acidic protons) strongly exhibit the ability to dissolve cellulose [60]. In contrast, weak hydrogen-bonding anions, e.g., BF4−, PF6−, and Tf2N−, are not suitable for cellulose dissolution [61,62]. The Cl−-based ILs are widely adopted as a small-sized hydrophilic hydrogen bond acceptor, for instance BmimCl is frequently used due to its thermoplasticity and thermal processability [63,64,65,66]. The EmimAc provides better solubility for cellulose due to its lower viscosity (~140 mPas at 25 °C and 10 mPa s at 80 °C) and to the fact that it forms easily processable and stable cellulose solutions; it is also less toxic (LD50 > 2000 mg/kg) and less corrosive than BmimCl [67,68,69,70]. Therefore, it has been a choice for creating regenerated cellulose hydrogels, especially in the form of fibers, because it requires less energy during the shaping process [71]. The AmimCl is also able to dissolve cellulose at a higher concentration than BmimCl [55,72], owing to its high Cl− concentration, electrochemical stability, and compatibility of hydrogen bond acceptors providing more interaction sites for hydroxyl groups of cellulose [73,74]. AmimCl is an effective IL for cellulose dissolution and hydrogel fabrication. The regenerated cellulose produced from AmimCl has the highest crystallinity, tensile strength, and transparency compared with those from BmimCl and EmimAc [72]. Even though AmimCl shows greater cellulose dissolution capability than EmimDEP [75], the content of depolymerized cellulose increases more with time and temperature in AmimCl than in EmimDEP [75]. Xu et al. [76] studied Amim(MeO)PHO2 IL for cellulose dissolution and decomposition. To increase the ionicity of this IL, Na2PHO3 inorganic salt, having an anion structure similar to that of Amim(MeO)PHO2, was added to form a composite solvent system (Na2PHO3/Amim(MeO)PHO2) and facilitate cellulose dissolution. The Na2PHO3 addition reduced H+ concentration of the solvent system, which inhibited the decomposition of cellulose. The lower viscosity of Na2PHO3/Amim(MeO)PHO2 solvent caused 22% w/w cellulose to dissolve at 80 °C over 48 h. With the increase in Na2PHO3 content, the degree of polymerization and thermal stability of the regenerated cellulose increased, but the crystallinity decreased relative to that of the original microcrystalline cellulose (MCC), because the long molecular chains exhibited difficulty in forming new hydrogen bonds during regeneration [76].
One of the advantages of imidazolium-based ILs is their capability to dissolve cellulose even at room temperature. However, some drawbacks are the high viscosity and difficulty in cellulose dispersion due to the strong association between the anions and cations [77]. When ILs are used in the presence of lignin in the pulp or impurities, side reactions such as acetylation may occur at high temperatures above 100 °C [78]. Therefore, the researchers have studied new ILs for efficient cellulose dissolution. Li et al. [79] developed a series of superbase-derived ILs such as: 1,8-diazabicyclo [5.4.0] undec-7-ene (DBU) including 1,8-diazabicyclo [5.4.0] undec-7-enium (DBUH) carboxylate, e.g., OAc−; and 1,5-diazabicyclo [4.3.0] non-5-ene (DBN) including 1,5-diazabicyclo [4.3.0] non-5-enium (DBNH) carboxylate with lower viscosity and high cellulose solubility. The DBUH ethoxyacetate has the higher cellulose dissolution capacity (14.8% w/w wood pulp at 80 °C) [80], but lower corrosion to steels than EmimAc and AmimCl [81]. On the other hand, some organic N-oxide-based solutions can dissolve cellulose efficiently, but the drawbacks are the instability of the N-oxides and the high dissolution temperature leading to the partial degradation of cellulose. Lui et al. [82] prepared DBN coupled with two N-oxides, i.e., pyridine N-oxide (PyO) and 2-picoline-N-oxide (PiO). The solvents dissolved cellulose efficiently at temperatures lower than 80 °C. The solvent DBN/PyO, at molar ratio of DBN and PyO of four, showed the cellulose solubility of 10.9 and 14.1% w/w at 50 and 70 °C, respectively. Galamba et al. [83] studied benzethonium- and didecyldimethylammonium- based ILs combined with short alkyl carboxylate anions to dissolve MCC at a concentration of 4% w/w. The polymeric cellulose hydrogel structures varied depending on the type of ILs and the ratios between cellulose and IL. However, after regeneration, some ILs remained in the hydrogel structures, which was advantageous in terms of the antibacterial/antimicrobial response of the hydrogels. Some new IL systems for cellulose dissolution, as reported in recent studies, are shown in Figure 1.
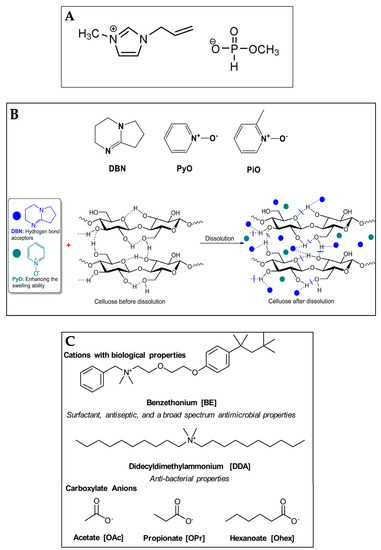
Figure 1. Chemical structures of 1-allyl-3-methylimidazolium methyl phosphonate (Amim (MeO)PHO2) (A), 1,5-diazabicyclo [4.3.0] non-5-ene (DBN), pyridine N-oxide (PyO) and 2-picoline N-oxide (PiO) with graphic dissolution mechanism of cellulose (B) and benzethonium (BE)- and didecyldimethylammonium (DDA)-based ILs combined with short alkyl carboxylate anions (C).
2.2. Ionic Liquid–Cosolvent Systems
One of the problems with using ILs to process cellulose is the high viscosity of the cellulose/IL solutions. The use of IL/cosolvent mixtures is an option that can modify the properties of the IL solvent by improving the rheological properties of the cellulose mixture, to reduce the viscosity of the solvent system and to accelerate the mass transfer process. The solvation of cation and anion by the cosolvents enhances the production of more free anions from anion–cation pairs, which readily interact with cellulose [84,85]. The frequently used cosolvents are polar aprotic organic solvents like DMSO, dimethylformamide (DMF), dimethylacetamide (DMAc), and dimethylimidazolinone (DMI). Zhou et al. [86] studied the cellulose solution state and formation mechanism by using these four cosolvents with EmimAc, BmimAc, AmimCl, and BmimCl. Acetate-based ILs and a high cosolvent content facilitated the formation of a molecularly dispersed state. In contrast, a state of coexistence of single molecular chains and undissolved cellulose microdomains gradually converted into a molecularly dispersed state when using chloride based ILs or a low content of cosolvents. The cosolvents DMF and DMAc promoted the molecular dispersion of cellulose better than DMSO and DMI. In IL/cosolvent systems with a high hydrogen-bonding basicity (>0.92) and a large amount of small ion cluster structures, cellulose favors the formation of a molecularly dispersed state. Cellulose dissolution capacity can be increased at lower processing temperature. Importantly, this IL/cosolvent system reduce the quantity of IL used in cellulose dissolution [87]. Other recent studies also indicate the advantages of the mixed IL/cosolvent system for cellulose dissolution that time, temperature, and viscosity of cellulose mixture in IL solutions are reduced by the inclusion of a cosolvent [88,89]. Hawkins et al. [88] studied the dissolution time of flax fibers in EmimAc as a function of temperature and DMSO concentration. The dissolution rate was maximized when using an equal amount of EmimAc to DMSO, whereby the dissolution was about three times faster than that in the pure IL. The independence of an activation energy (Ea), required for the dissolution of flax fibers in EmimAc, on DMSO concentration suggests that flax fibers dissolution is not primarily a viscosity-driven process. The result is related to the work of Chen et al. [90]; the dissolution is not only governed by viscosity, but also by solvent power.
Due to its low cost, low toxicity, and low viscosity, DMSO is a suitable candidate for a cosolvent. A series of IL/DMSO systems have been studied as shown in Table 1. The efficiency of IL/DMSO mixtures in cellulose dissolution and derivatization depends on the structures of ILs [91] and temperature [92]. With increasing temperature, the specific interaction between cellulose and the solvent increases [92]. Ferreira et al. [91] studied the effect of the presence of an ether linkage in the sidechain of imidazolium acetate (ImAc) using RMenImAc, R = 1-butyl or 2-methoxyethyl, n = 1 or 2 and the mixtures with DMSO. The mixture of BmimAc–DMSO was more efficient for the dissolution of MCC and acylation of cellulose because the mixture was less viscous, more basic, and formed stronger hydrogen bonds with cellobiose. Using the corresponding ILs with C2–CH3 instead of C2–H (1-butyl-2,3-dimethylimidazolium acetate and 1-(2-methoxyethyl)-2,3-dimethylimidazolium acetate) increased the concentration of dissolved cellulose [91]. Increasing the amount of DMSO does not only improve cellulose solubilization, but also leads to the formation of a more pronounced macroporous structure of the hydrogel [93].
Table 1. Recent studies on IL/DMSO solvent systems used for cellulose dissolution.
| IL | Concentration of DMSO in IL and Temperature to Dissolve Cellulose | Concentration of Cellulose | Ref. |
|---|---|---|---|
| C3OMeImAc C4MeImAc (BmimAc) C3OMe2ImAc C4Me2ImAc |
60% mole, 60 °C 60% mole, 60 °C 60% mole, 60 °C 60% mole, 60 °C |
12% w/w MCC a 16% w/w MCC a 19% w/w MCC a 22% w/w MCC a |
[91] |
| BmimAc | 20% w/w, 70 °C | 8% w/w MCC, Avicel, and α-cellulose b | [93] |
| 40% w/w, R.T. c | 20% w/w Avicel b | [94] | |
| 40% w/w, R.T. c | ~10% w/w MCC b | [95] | |
| 75% w/w, 80 °C | 14% w/w cellulose powder b | [96] | |
| BmimCl | 50% w/w, 15–75 °C | 3% w/w cellulose powder b | [92] |
| 30% w/w, 120 °C | - | [97] | |
| EmimAc | 50% w/w, 60 °C | 2.5% w/w cotton b | [98] |
| 25% w/w, 50 °C | 10% w/w MCC b | [99] | |
| 75% w/w, 80 °C | 14% w/w cellulose powder b | [96] | |
| EmimCl | 75% w/w, 80 °C | 14% w/w cellulose powder b | [96] |
In designing a new IL solvent system for cellulose dissolution, reversible/switchable reactions with CO2, a well-recognized greenhouse gas, have been extended [100,101,102]. Xie et al. [100] reported that the CO2-based reversible IL/DMSO solvent could dissolve MCC up to 10% w/w under mild conditions at 60 °C and a CO2 pressure of 2.0 MPa for 2 h, when 1,1,3,3-tetramethyl guanidine (TMG) was used as a base, in conjunction with methanol, ethanol, propanol, butanol, and ethylene glycol (Figure 2A). Yang et al. [103] also reported that DBU/DMSO could promote the dissolution of cellulose pulp at the relative low temperature of 50 °C in the presence of CO2. This is because the addition of CO2 causes the reversibility or switch-ability of this solvent system by changing the polarity from nonpolar to polar and reversing to its initial nonpolar state upon the release of CO2 [104,105]. Cellulose dissolves in this solvent system by nonderivative and derivative approaches (Figure 2B). In the nonderivative approach, simple alcohols such as methanol, hexanol, or ethylene glycol, in the presence of strong organic bases such as DBU, react with CO2 leading to the formation a DMSO–carbonate species solvent that can solubilize cellulose [100]. In the derivative approach, the superbase activates cellulose first and, thereafter, reacts with CO2 to form a DMSO-soluble cellulose carbonate intermediate [106].
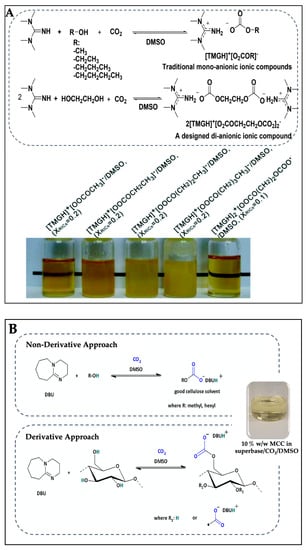
Figure 2. Reaction of 1,1,3,3-tetramethyl guanidine (TMG) with methanol, ethanol, propanol, butanol, and ethylene glycol in the presence of CO2 and dimethyl sulfoxide (DMSO) to form reversible ILs (TMGHO2COR) (A). Pictures illustrate 5% w/w of microcrystalline cellulose (MCC) solution in the reversible ionic compound (RIC) solvents at the different molar fractions in DMSO after CO2 capture (XRIC). Nonderivative and derivative approaches of the CO2 switchable 1,8-diazabicyclo [5.4.0] undec-7-ene (DBU) super base solvent system (B). Picture illustrates 10% w/w of MCC solution in superbase (DBU)/CO2/DMSO solvent.
Since the reversible reaction between cellulose, organic base, and CO2 is exothermic, the resulting high temperature is not suitable for cellulose dissolution. Therefore, the temperature used in most studies is in the range of 30–60 °C. At 30 °C, increasing the pressure of CO2 above 20 bar (2 MPa) increases in the carbonate formation and cellulose solubilization kinetics in 10 min, whereas at 40 bar (4 MPa), the carbonate formation is reached within 5 min. On the other hand, for CO2 pressures below 2 MPa, the solubilization of 3% w/w MCC is slower but occurs within 15 min [107]. At 30 °C and CO2 pressures of 0.2 MPa, the solubility of MCC in DMSO/DBU at weight ratio of DBU of 0.1 can reach 9% w/w. However, increasing the weight ratio of DBU to 0.5 slightly inhibits the MCC solubility. This indicates that the DBU amount influences MCC dissolution behavior [108]. At the higher temperature range of 40–60 °C, the dissolution of cellulose derived from a cassava pulp waste in DBU and ethylene glycol, which produces a switchable polarity solvent containing the cation of DBU (DBUH+) and the anion of ethylenedicarbonate (O2COCH2CH2OCO22−) in the mixture with CO2, can be achieved in less than 30 min under CO2 pressure of more than 5.0 MPa [109]. Under 1 atm (0.1 MPa) in an open system, the obtained cellulose is stable and suitable for the preparation of cellulose hydrogel fiber by wet spinning [110]. Besides TMG and DBU, clear cellulose solutions were obtained in 1,5,7-Triazabicyclo [4.4.0] dec-5-ene (TBD) under 1 MPa CO2 at 50 °C for 3 h [111], and 2-tert-butyl-1,1,3,3-tetramethylguanidine (BTMG) under 1 atm (0.1 MPa) CO2 at room temperature for 5 min [112], but cellulose was not dissolved in triethyl amine (TEA) [111]. Instead of DMSO, other organic solvents with low Henry’s constants, such as propylene carbonate and sulfolane, are used as CO2 absorbents to increase CO2 absorption capacity in the solvent, so that the cellulose dissolution is enhanced. With the addition of propylene carbonate, the paper cellulose dissolution can be increased from 1.0 to 5.0% w/w, while those for microcrystalline cellulose and corncob cellulose are increased from 5.0 to 10.0 and 7.0% w/w, respectively, at 60 °C and a CO2 pressure of 0.5 MPa. The chemical and crystalline structures of the regenerated cellulose is not affected by the addition of these CO2 absorbents [113].
This entry is adapted from the peer-reviewed paper 10.3390/gels9070546
This entry is offline, you can click here to edit this entry!
