These are classified depending on (a) histopathological grade and proliferative (mitotic) activity, by the World Health Organization (WHO) into (A) well-differentiated grade I, II, and III neuroendocrine tumour (NET) with proliferative index and mitotic activity (< 3%, <2%; 3 to 20%, 2 to 20% and >20%, respectively) and (B) poorly-differentiated neuroendocrine carcinoma (NEC) with mitotic and Ki-67 indices >20% and poorly differentiated histology. The 2019 WHO classification of NET has also divided NENs as NET and NEC based on genetic mutations: mutations in
MEN1,
DAXX, and
ATRX are entity-defining for well-differentiated NETs, whereas NECs usually have
TP53 or
RB1 mutations [
7,
8]; (b) functioning status depending upon secretion or non-secretion of peptide hormones as functioning and non-functioning NENs; and (c) embryological origin as foregut, mid-gut, and hind-gut derivatives.
The diagnosis of NET is characterized by detection of immunohistochemical markers—synaptophysin, chromogranin A, and neuron-specific enolase. Over the years, the role of nuclear medicine has grown to play a central role in the diagnosis of NEN after the identification of somatostatin (SST) in 1973 and 5 types of somatostatin receptors (SSTRs 1 to 5) in the early 1990s. The discovery of somatostatin receptors has opened up new avenues for diagnosis, staging, and treatment of NENs, especially the well-differentiated type. Somatostatin-receptor-based imaging using synthetic somatostatin agonists (SSA) was started in 1994 with [
111In] In-pentetreotide (Octreoscan) being the first Food and Drug Association (FDA) approved and commercially marketed radiopharmaceutical [
9,
10,
11,
12]. Although there was wide acceptance of Octreoscan for NEN imaging, the radiopharmaceutical had many limitations—less favorable tumor-to-background ratio, moderate affinity for receptors, and high gamma energy causing more background noise and high radiation absorbed dose to the patient. To a great extent, these limitations have been alleviated with the advent of the next generation of SSA labelled with positron emitter radio-metal [
68Ga] to be used with PET-CT [
13,
14]. Recent advancements in the detection and mapping of SSTR expression in vivo has opened avenues for targeting the same for therapeutic benefits and personalized management. After securing FDA approval in January 2018, peptide receptor radionuclide therapy (PRRT) using Lutetium-177 DOTA-TATE ([
177Lu]Lu-DOTA-TATE) has gained widespread acceptance as one of the frontline treatments in metastatic/inoperable neuroendocrine tumors (NET).
2. Radiopharmaceuticals for SSTR-PET Imaging
2.1. Somatostatin Receptor Agonists
Somatostatin receptors are G-protein coupled receptors (GPCR) binding to somatostatin neuropeptides, a paracrine secreted by gastro-intestinal and brain cells. Presently, various types of somatostatin agonists and few antagonists are available for clinical and/or experimental use. The common radio-pharmaceuticals for clinical use are–[
68Ga]Ga-DOTA-Tyr3-Octreotate (DOTA-TATE), [
68Ga]Ga-DOTA-Phe1-Tyr3-Octreotide (DOTA-TOC), and [
68Ga]Ga-DOTA-NaI3-Octreotide (DOTA-NOC). These three radiopharmaceuticals differ slightly in their pharmacokinetic properties, mainly due to different affinities for SSTR subtypes; while DOTA-TATE is SSTR 2 specific, DOTA-NOC has affinity towards SSTR 2, 3, and 5 and DOTA-TOC has affinity towards SSTR 2 > 5 [
15,
16,
17,
18]. Despite different receptor affinity, there is no clinically significant difference and there are ample data to support high accuracy of SSTR PET-CT for detecting lesions as compared to conventional imaging and somatostatin-receptor scintigraphy [
19,
20,
21,
22,
23,
24,
25].
[18F]-Fluorine (
18F) labelled radio pharmaceuticals are recently being developed with the following advantages: long half-life, no need of in-house generators or cyclotron, and relatively low positron energy which leading to better spatial resolution than [
68Ga]. Al [18F]F-NOTA-Octreotide is one recent promising radiopharmaceutical demonstrating high affinity for SSTR2, favorable biodistribution, high tumor uptake, better spatial resolution, and is proven to be safe for clinical applications [
5,
26,
27,
28,
29,
30,
31].
[
64Cu]Cu-DOTATATE is a cyclotron produced positron emitter that can be manufactured on a large scale, yields similar detection rates as [
68Ga]-based SSTR-PET agents and has better pharmacokinetic properties such as (i) longer half-life (~12.7 h), (ii) relatively low positron energy (0.65 vs. 1.9 MeV) leading to shorter positron range (mean −0.56 vs. 3.5 mm), and (iii) higher spatial resolution enabling better detection of smaller lesions. [
64Cu] hence permits delayed serial imaging with important implications for personalized dosimetry in peptide receptor radionuclide therapy (PRRT) and radio-guided surgery using a hand-held positron probe [
32,
33].
2.2. Somatostatin Receptor Antagonists
In opposition to the general belief that agonists will be more suitable as an imaging agent since they are internalized, a recent in vitro study using SSTR-3 antagonist has demonstrated that the antagonist detected 76-fold more sites of binding as against SSTR-3 agonist [
34]. Few recent studies have shown that radiolabeled SSTR antagonists produce superior images than radiolabeled SSTR agonists [
34,
35]. Recently, few studies with small number of NET patients demonstrated radiolabeled SSTR-2 antagonists, e.g., [
111In]In-DOTA-BASS and [
68Ga]Ga-OPS202 ([
68Ga]Ga-NODAGA-JR11) have demonstrated superior images and higher sensitivity compared to radiolabeled SSTR-2 agonists [
34,
35,
36,
37,
38]. This led to the opinion that [
177Lu] labelled antagonist in PRRT may be utilized instead of [
177Lu]-labelled agonists. [
177Lu]Lu-DOTA-JR11 provided 1.7-to-10.6-fold higher tumor uptake as compared to agonists, resulting in partial remission in half of the enrolled patients [
35].
2.3. Appropriate Use Criteria
Appropriateness use criteria (AUC) defines set of scenarios finalized by the representatives of international societies concerned with management of NETs. The process of determining AUC was modelled after RAND/UCLA appropriateness method including a list of common scenarios encountered in NET management, a systematic review of evidence related to these scenarios and development of an appropriateness score for each scenario using a modified Delphi process [
39,
40]. The workgroup identified 12 scenarios for patients with NETs and scored each scenario as “appropriate”, “may be appropriate”, or “rarely appropriate” on a scale from 1 to 9; where 7 to 9 are appropriate, 4 to 6 are considered may be appropriate, and 1 to 3 indicate that the use is rarely appropriate and is not considered acceptable.
- (a)
-
Appropriate: Initial staging after histologic diagnosis of NET-9; localization of primary tumor in patients with known metastatic NET but unknown primary-9; selection of patients for SSTR targeting PRRT-9; staging NETs before planned surgery-8; evaluation of mass suggestive of NET and not amenable to endoscopic or percutaneous biopsy-8; monitoring of NETs seen predominantly on SSTR PET-8; evaluation of patients with biochemical evidence and symptoms of NET without evidence on conventional imaging (CI) and without prior histological diagnosis of NET-7; restaging at the time of clinical or laboratory progression without progression on CI-7; and new indeterminate lesion on CI with unclear progression-7.
- (b)
-
May be appropriate: Restaging of patients with NETs at initial follow up after resection with curative intent-6; selection of patients with non-functional NETs for SSA treatment-6; and monitoring in patients with NETs seen on both CI and SSTR PET with active disease and no clinical evidence of progression-6.
2.4. Clinical Utility of SSTR-PET
Detection and Initial Staging: When compared with conventional imaging, SSTR-PET offers multiple advantages in terms of detection rate of primary and metastatic disease and change in management (observed in 44% upfront and in 9% of patients who have undergone previous somatostatin receptor scintigraphy with Octreoscan) ([
41],
Figure 1). Bauckneht et al., in their review and meta-analysis including 1143 patients of pancreatic NET, demonstrated a pooled sensitivity and specificity of 79.6% (95% CI—71 to 87%) and 95% (95% CI—75 to 100%), respectively; heterogeneity of 59.6% and 51.5%, and on a per patient and per lesion basis, the pooled detection rates of primary lesion were 81% (95% CI—65 to 90%) and 92% (95% CI—80 to 97%), respectively [
42]. Geijer and Breimer, in their meta-analysis on 2015 patients, demonstrated pooled sensitivity of 93% (95% CI—91 to 94%) [
43]. Despite theoretical advantage, conflicting results were observed in detection with contrast enhanced vs. non-enhanced PET-CT for the detection of primary and metastatic disease. While Kazmierczak et al. showed 50% and 30% improvement in sensitivity and accuracy, Mayerhoefer et al. found only moderate improvement in sensitivity and hardly any change in specificity [
44,
45]. Similarly, combining PET and MRI has theoretical advantage of high soft tissue contrast for MRI and metabolic data from PET.
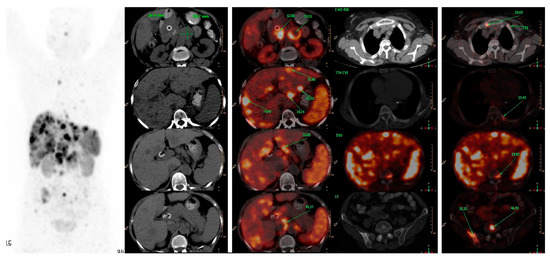
Figure 1. A 66-years-old female patient, presented with features of obstructive jaundice. Triphasic CECT scan abdomen showed large hypodense SOL in head of pancreas encasing CBD with significant upstream dilatation of biliary tree with multiple hypodense bilobar hepatic metastases and multiple abdominal & retroperitoneal lymph nodes. Patient was referred for PRRT and 68Ga-DOTATATE PET-CT was done which confirmed above-mentioned triphasic CE-CT scan findings and showed many new skeletal and marrow lesions at multiple skeletal sites. Hence is SSTR-based PET-CT represents a better modality for metastatic workup than conventional imaging.
Detection of unknown primary: Carcinoma of unknown primary (CUP) accounts for 3 to 5% of all malignancies and is divided into following subtypes based on histological subtypes: adenocarcinoma (80 to 85%), squamous cell carcinoma (5 to 10%), and neuroendocrine tumor (2 to 4%) [
46]. Neuroendocrine tumors of unknown primary (CUP-NETs) are primary tumors with undetermined origin among metastatic NETs and accounts for 11 to 22% of NETs ([
47,
48];
Figure 2). Ma et al., in their meta-analysis of 484 patients, demonstrated pooled sensitivity and specificity of SSTR imaging in identifying CUP-NETs as 82% and 55%, respectively [
49]. The area under the receiver operating curve (ROC) was 69% and pooled detection rate for CUP-NETs was 61%. SSTR PET-CT identified most metastases in liver (57.9%) followed by lymph nodes (22.8%), bones (12.8%), lung (2.8%), and others (1.7%). Sampathirao et al., in their study on 51 CUP-NET patients using “dual tracer” PET-CT with [
68Ga]GA-DOTATATE and [
18F]-FDG, demonstrated sensitivity of 60.78%, whereas overall lesion detection sensitivity was 96.87% [
50]. Delpassand et al., in their study, found [
64Cu]Cu-DOTATATE to be an effective radiopharmaceutical in detecting NET lesions with sensitivity and specificity of 90.9% and 96.6%, respectively [
32].
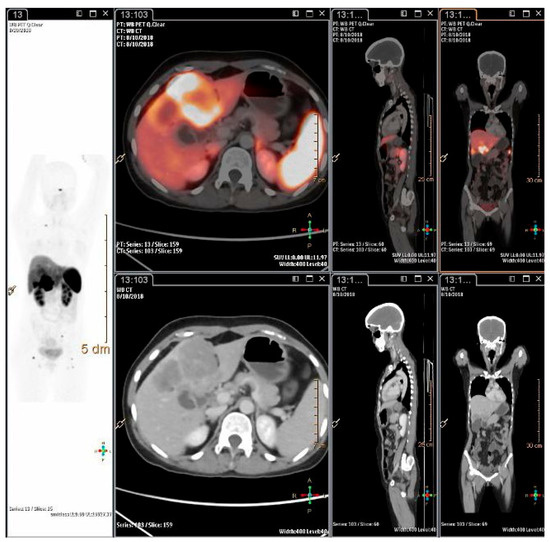
Figure 2. A 26-years-old lady, presented with pain abdomen and was evaluated for the same. CECT abdomen showed multiple liver lesions and abdominal lymph nodes. Biopsy and IHC from liver lesion showed high grade neuroendocrine carcinoma, large cell variant (Ki-67 index 80%). She was referred for opinion for PRRT. 68Ga-DOTATATE PET-CT scan showed intensely SSTR expressing polypoidal gall bladder mass infiltrating into inferior surface of liver with SSTR expressing multiple liver lesions, abdomino-pelvic lymph nodes and SSTR expressing lesion in head of right femur.