Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Phlorotannins are moderately hydrophilic components with a wide range of molecular weights, ranging between 126 and 650 kDa. They are produced via the polymerization of the phloroglucinol molecule (benzene-1,3,5-triol) through the polyketide pathway reaction and stored in physodes and/or cell-wall-forming complexes.
- phlorotannins
- bioactivities
- solid–liquid extraction
- ultrasound-assisted extraction
- solvent
1. Introduction
The content of phlorotannins in seaweeds depends on environmental conditions, such as tides, salinity, light availability, UV radiation, and herbivory intensity. These compounds are divided into six different classes according to the variations in their assemblage and distribution of hydroxyl groups: eckols, fuhalols, fucophlorethols, phlorethols, fucols, and carmalols (Figure 1). Phlorethols and fuhalols present aryl–ether linkages, fucols aryl–aryl bonds, fucophlorethols, and a mixture of ether and phenyl bonds; eckols present a 1,4-dibenzodioxin unit. Carmalols present a 4-dibenzodioxin unit at the third and seventh positions. Eckols differ from carmalols in their lower molecular weight and the presence of an OH group substituted at the fourth carbon; fuhalols differ from phlorethols in their regular sequence of para- and ortho-ether bonds, the presence of additional OH groups in every third ring, and the lack of one or more OH groups in the whole molecule [1][2][3].
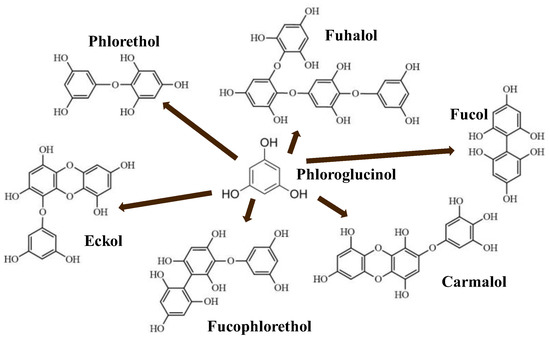
Figure 1. Phlorotannin main groups derived from phloroglucinol polyketide pathway reaction.
The bioactivities and characteristics of phlorotannins as well as their amount are influenced by the extraction method used and the employed conditions (e.g., operation mode, solvent, solid–liquid ratio, time, temperature, and pre- and post-treatments) [4][5]. For instance, the selection of the operation mode is a key aspect of industrial production. Batch extraction requires interruptions for charging, discharging, and cleaning steps and high amounts of solvent. These problems are reduced under semicontinuous extraction consisting of several batch extractors operated in series. Another important factor is the solvent used for the extraction to achieve high operation yields and minimize the coextraction of undesirable substances. Indeed, the extraction of phlorotannins is a challenge because they are deeply embedded among the seaweed components, forming complexes [6]. In addition to the solvent, phlorotannins’ solubility is influenced by the polymerization degree and interactions with other food constituents [7][8]. Despite organic solvents being largely recommended for the extraction of antioxidants from plants and seaweeds in terms of extraction yield, these solvents are volatile, inflammable, and/or toxic [9][10][11]. As an alternative, water is being proposed as an efficient and green-labeled alternative for polyphenol extraction from seaweed [4][12][13]. Here, several extraction methods have been tested to extract bioactive compounds from algal material, aiming to develop new, safe, effective, and affordable extraction technologies to minimize the presence of residues. Moreover, in recent years, there has been growing interest in developing greener and cleaner extraction technologies. These methods (Figure 2) are being extensively studied to ensure that they are not only effective but also environmentally friendly and sustainable [3][14][15].
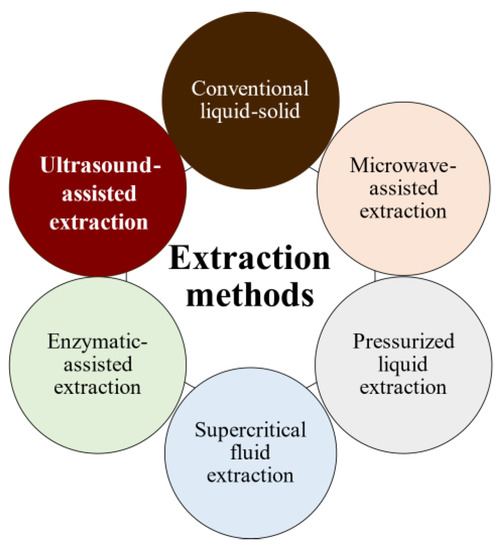
Figure 2. Phlorotannins’ extraction methods.
Pressurized liquid extraction (PLE) utilizes high pressures (up to 15 MPa) and temperatures (up to 200 °C), along with low extractant volumes and short extraction times, whereas microwave-assisted extraction (MAE) uses electromagnetic waves to induce and facilitate compound extraction. However, both methods promote the partial degradation of thermolabile compounds [15]. Supercritical fluid extraction uses fluids with a temperature and pressure above their critical point (most often CO2) to increase mass transfer by decreasing surface tension and viscosity, but it is less used because of the high costs of equipment and solvent (if no solvent is recovered); additionally, for optimal results, the solvent polarity must be tuned by adding polar compounds (alcohols) to CO2 for phenolics extraction [16].
During phlorotannins extraction, other biomolecules are coextracted, mainly polysaccharides and proteins; hence, the separation and purification for the fractionation and/or isolation of desired compounds are recommended. Extract fractionation consists of separation based on molecular weights, charges, chemical affinities, and/or solubilities [17][18]. Adsorption-based separation methods, such as flash chromatography, are emerging among the fractionation techniques due to their simplicity, the potential for scale-up, and higher specificity compared with those of other primary fractionation techniques [18]. Separation is achieved by the contact of seaweed extracts with a solid matrix with different affinities for phlorotannins and the remaining compounds. Then, phlorotannins can be recovered by separating the solid and liquid phases. In addition to solid-phase extraction, in which the sorbent is immobilized on a cartridge or column, allowing sequential elution of compounds with a solvent gradient, liquid–liquid extraction is a solubility-based separation method in which the wide range of polarities of phenolic compounds allows their relatively easy partitioning. Another possibility is based on ultrafiltration and/or molecular-weight cut-off dialysis. These techniques require minimal instrumentation and expertise and allow the quick separation of fractions over a wide range of molecular weights using only a few combinations of membranes or filters. Two extraction methods have been extensively studied for extracting phlorotannins: conventional solid–liquid extraction (SLE) and ultrasound-assisted extraction (UAE).
2. Solid–Liquid Extraction
Conventional SLE, also known as leaching, is a widely used method in the food industry to extract various compounds such as sucrose, lipids, proteins, phenolic compounds, and hydrocolloids [4][19][20]. Molecular diffusion is the primary transport mechanism in this process, where compounds are transported by a concentration gradient in the solid phase. The microstructure of the solids plays a significant role in the rate and characteristics of the extraction process due to factors such as porosity, pore size, and moisture content. The microstructure refers to the arrangement and characteristics of the solid material at the microscopic level, including the distribution and size of pores or void spaces within the material. SLE is a complex process involving multicomponent, multiphase, and unsteady-state mass transfer operations, which are affected by the relative migration rate of different compounds through the solid. The SLE process can be divided into several stages that can occur simultaneously or, in some cases, sequentially: (1) solvent entrance into a solid matrix; (2) solubilization/breakdown of components; (3) solute transport to the solid matrix surface; (4) extracted solute migration from the solid surface through the solvent bulk; (5) separation of the extract and solid. Understanding each of these stages is crucial for optimizing the SLE process and improving the extraction yield. Compound transport through the solid matrix is usually the rate-controlling step, resulting in an energy- and time-consumption process that requires potentially high amounts of solvents to produce acceptable extraction yields [21]. The extraction of bioactive compounds at a commercial scale requires high extraction yields and the conservation of their bioactivities, which are difficult to achieve using the conventional SLE method [22]. The simultaneous processes involved in the SLE of compounds from seaweed cells are shown in Figure 3.
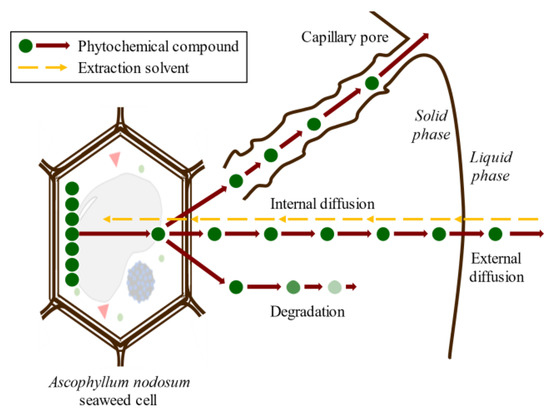
Figure 3. Scheme of the simultaneous stages of SLE of compounds from seaweed cell.
3. Ultrasound-Assisted Extraction
Ultrasound technology is widely used in the food industry because it can be used either as a pretreatment or can be combined with different types of solvents [23][24]. Ultrasound comprises mechanical waves, ranging from 20 kHz up to 10 MHz, involving various phenomena such as shear forces, compression pressure gradients, agitation, rarefaction, vibration, microjets, radical formation, and cavitation [14]. UAE utilizes soundwaves to disintegrate the cell structure for the subsequent release of compounds. Cavitation is the main force driving ultrasound extraction: it is a hydrodynamic effect that occurs when vapor cavities are created within the liquid, and different pressure forces are present [25]. These processes produce the expansion and implosive collapse of microbubbles, formed via a series of compressions and rarefactions in molecules generated by ultrasound waves, improving heat and mass transfer along the system and improving solvent penetration and cell-wall breaking. The main advantages of UAE are its reduced solvent consumption, shorter extraction time, lower operational costs, minimal impact on the stability of the target compounds because high temperatures are not required, and higher process efficiency and extraction yields compared with conventional extraction methods [14][26]. Figure 4 shows the general scheme of ultrasound-assisted extraction from seaweed tissues.
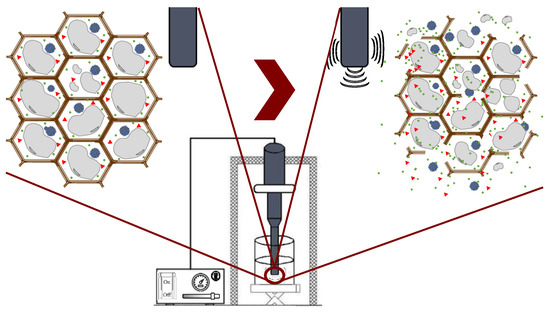
Figure 4. Ultrasound-assisted extraction process.
In the last twenty years, several researchers have studied SLE and UAE features, such as solvent type, liquid–solid ratio, time, and temperature as critical aspects of phlorotannin extraction from Ascophyllum nodosum brown seaweeds. However, there are limited data on the effect of using UAE to produce phenolic-compound-enriched extracts [27]. Phlorotannins are measured as total polyphenol content (TPC) based on the Folin–Ciocalteau method. Additionally, different standards have been used for quantification of, for example, gallic acid, phloroglucinol, pyrocatechol, raw phlorotannin, and catechin. The establishment of a standardized protocol to quantify algal antioxidant extract activity would be convenient. The main studies are summarized in Table 1. According to the reviewed literature, the TPC values have ranged from 0.7 mgPE/mg extract [19] to 0.5 g PE/g extract [19], working with ethanol at 96% and 40% v/v, respectively. Furthermore, the lowest TPC values were obtained using SLE, with a high liquid–solid ratio (LS = 90 g solvent/g d.b) and an intermediate operational time (t = 30 min) and temperature (T = 30 °C). On the contrary, the highest extraction was achieved using UAE, with a lower LS value (50 g solvent/g dry basis (d.b)), similar time (30 min), and higher temperature (60 °C). According to the literature, organic solvents have been commonly used for extracting phlorotannins, the extraction procedures have shown increased extraction yields, and low temperatures have typically been used to prevent the thermal degradation of phytochemicals.
Table 1. Overview of extraction conditions (method, extractant, liquid–solid ratio, time, and temperature) to obtain phlorotannins-enriched extracts from Ascophyllum nodosum.
| Method | Extractant | LS (gsol/galgae) |
Time | Temperature | TPC | Reference |
|---|---|---|---|---|---|---|
| SLE | Ethanol | 90 | 30 min | 30 °C | 0.7 mgPE/mgextract | [20] |
| SLE | Water | 100 | 1 h | 65 °C | 7.3 mgPE/gextract | [28] |
| Ethanol (30% v/v) | 5 | 30 min | 25 °C | 4.1 mgPE/gextract | ||
| Ethanol (80% v/v) | 10 | 20 (+5) h | 25 (+65) °C | 3.4 mgPE/gextract | ||
| SLE | Acetone (70% v/v) | 20 | 3 h | rt | 24.5 mgPE/gextract. | [1] |
| SLE | Ethanol (50% v/v) | 15 | 4 h | 20 °C | 0.2 gPE/gextract | [27] |
| UAE | 10 | 30 min | NS (35 kHz) | 0.4 gPE/gextract | ||
| UAE | Ethanol (40% v/v) | 50 | 30 min | 60 °C | 0.5 gPE/gextract | [19] |
| SLE | Water | 10 | 24 h | 4 °C | 52 mgPE/gextract | [29] |
| Methanol (50% v/v) | 77 mgPE/gextract | |||||
| Ethanol (75% v/v) | 95.4 mgPE/gextract | |||||
| Dioxolane (75% v/v) | 90 mgPE/gextract | |||||
| 1,3-propanediol | 98.5 mgPE/gextract | |||||
| UAE | Ethanol (50% v/v) | 10 | 30 min | rt | 46.6 mg/gextract | [30] |
| SLE | Methanol (70% v/v) | 10 | 4 h | rt | 0.5 gGAE/gextract | [31] |
| MAE (2.45 GHz) |
15 min | 110 °C | 1.4 gGAE/gextract | |||
| SLE | Methanol (70% v/v) | 10 | 4 h | rt | 0.5 mgGAE/gd.b | [32] |
| MAE (2.45 GHz) |
15 min | 110 °C | 1.4 mgGAE/gd.b | |||
| UAE (80 W/cm2) |
Acetone (70% v/v) | 30 | 4 min | <35 °C | 31.8 mgPE/gd.b | [33] |
| SLE | Water | 20 | 150 min | 70 °C | 0.17 mgPE/gd.b | [34] |
| HCl (0.1 M) | 0.11 mgPE/gd.b | |||||
| UAE (35.6 W/cm2) |
Water | 15 min | <35 °C | 0.16 mgPE/gd.b | ||
| HCl (0.1 M) | 0.13 mgPE/gd.b | |||||
| UAE (75.8 W/cm2) + SLE | HCl (0.03 M) | 10 | 10 min + 22 h |
<35 °C | 135.7 mgGAE/gd.b | [35] |
| UAE (75.8 W/cm2) |
HCl (0.03 M) | 10 | 25 min | <35 °C | 143.1 mgGAE/gd.b | [36] |
| SLE | Water | 10 | 60 min | 20 °C | 178.0 mgGAE/mL | [37] |
| HCl (5 mM) | 210.0 mgGAE/mL | |||||
| SLE | Water | 20 | 24 h | rt | 70.5 mgPE/gextract | [15] |
| Ethanol (80% v/v) | 10 | 66.3 mgPE/gextract | ||||
| Acetone (80% v/v) | 155.9 mgPE/gextract | |||||
| PLE | Water | 2 | NS | 120 °C (1500 psi) |
93.4 mgPE/gextract | |
| Ethanol (80% v/v) | 100 °C (1000 psi) |
101.3 mgPE/gextract | ||||
| Acetone (80% v/v) | 60 °C (1000 psi) |
127.4 mgPE/gextract | ||||
| SLE | Methanol (60% v/v) | 15 | 3 h | 40 °C | 4.5 mgGAE/gd.b | [38] |
| SLE | Ethanol (50% v/v) | 12.5 | 90 min | 80 °C | 38.8 mgPE/gd.b | [39] |
| SLE | Water | 20 | 24 h | rt | 138.0 mgPE/gextract | [40] |
| Acetone (70% v/v) | 159.0 mgPE/gextract |
Note: galgae, grams of algae; gd.b, grams of algae in dry basis (d.b); gsol, grams of solvent MAE, microwave-assisted extraction; mgextract, milligrams of extract; mgGAE, milligrams of gallic acid equivalents; mgPE, milligrams of phloroglucinol equivalent; NS, not specified in the study; rt, room temperature; SLE, solid–liquid extraction; UAE, ultrasound-assisted extraction; PLE, pressurized liquid extraction; TPC, total polyphenol content determined from the extract.
While many chromatographic techniques have been utilized for separating, isolating, purifying, identifying, and quantifying individual phenolic compounds from plant materials, research on individual phenolic compounds in brown algae remains limited. To enhance our understanding of the bioactive potential of brown-algae-derived phenolic compounds, we need to know the chemical and physical structure. Table 2 shows some of these studies regarding the phlorotannins isolated from brown seaweeds. The isolation of phlorotannins from different seaweed involves many steps, large solvent amounts, a long time, and large amounts of energy, making the process complicated and expensive. This explains the scarcity of standards and the current lack of commercial availability of phlorotannins. Nevertheless, the isolation of these compounds is required to understand their bioactivity for further use in real applications.
Table 2. Main phlorotannins isolated and identified from brown seaweeds and their chemical structure.
| Phlorotannin | Seaweed | Reference | |
|---|---|---|---|
| 2-Phloroeckol | 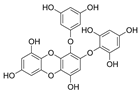 |
Eisenia bicyclis | [41] |
| Euphorbia stolonifera | [42] | ||
| 6,6′-Bieckol | 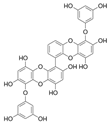 |
Ishige okamurae | [43] |
| 7-Phloroeckol | 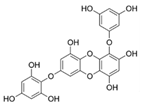 |
Euphorbia stolonifera | [42] |
| Ascophyllum nodosum | [44] | ||
| 8,8′-Bieckol | 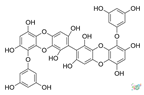 |
Eisenia bicyclis, Ecklonia cava and Ecklonia kurome |
[45] |
| Dieckol | 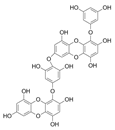 |
Eisenia bicyclis | [46] |
| Dioxinodehydroeckol | 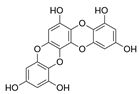 |
Euphorbia stolonifera | [42] |
| Diphlorethohydroxycarmalol | 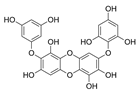 |
Ishige okamurae | [47] |
| Eckol | 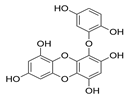 |
Ecklonia kurome | [48] |
| Fucodiphloroethol G | 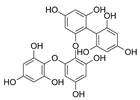 |
Ecklonia cava | [49] |
| Fucophlorethols | 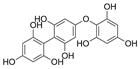 |
Cystophora retroflexa | [50] |
| Cupressus torulosa | [51] | ||
| Sargassum spinuligerum | |||
| Phlorofucofuroeckol A | 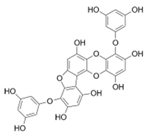 |
Ecklonia kurome | [52] |
| Euphorbia stolonifera | [53] | ||
| Phlorofucofuroeckol B | 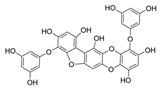 |
Erica arborea | [54] |
| Bifuhalol | 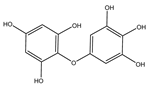 |
Bifurcaria bifurcata | [55] |
| Tetraphlorethols E | 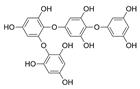 |
Cystophora retroflexa | [50] |
| Triphlorethol | 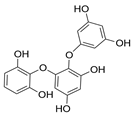 |
Ecklonia cava | [56] |
This entry is adapted from the peer-reviewed paper 10.3390/molecules28134937
References
- Ford, L.; Theodoridou, K.; Sheldrake, G.N.; Walsh, P.J. A critical review of analytical methods used for the chemical characterisation and quantification of phlorotannin compounds in brown seaweeds. Phytochem. Anal. 2019, 30, 587–599.
- Ragan, M.A.; Glombitzka, K.W. Phlorotannins, brown algal polyphenols. Prog. Phycol. Res. 1986, 4, 129–241.
- Kumar, L.R.G.; Paul, P.T.; Anas, K.K.; Tejpal, C.S.; Chatterjee1, N.; Anupama, T.K.; Mathew, S.; Ravishankar, C.N. Phlorotannins-bioactivity and extraction perspectives. J. Appl. Phycol. 2022, 34, 2173–2185.
- Leyton, A.; Pezoa-Conte, P.; Barriga, A.; Buschmann, A.H.; Maki-Arvela, P.; Mikkola, J.P.; Lienqueo, M.E. Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. Algal Res. 2016, 16, 201–208.
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and identification of phlorotannins from the brown alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49.
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331.
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014.
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of phlorotannins extraction from Fucus vesiculosus and evaluation of their potential to prevent metabolic disorders. Mar. Drugs 2019, 17, 162.
- Singh, I.P.; Sidana, J. Phlorotannins. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Functional Ingredients from Algae for Foods and Nutraceuticals; Dominguez, H., Ed.; Woodhead Publishing: Cambridge, MA, USA, 2013; pp. 181–204.
- Lopes, G.; Andrade, P.B.; Valentão, P. Phlorotannins: Towards new pharmacological interventions for diabetes mellitus type 2. Molecules 2017, 22, 56.
- Hermund, D.B.; Plaza, M.; Turner, C.; Jonsdottir, R.; Kristinsson, H.G.; Jacobsen, C.; Nielsen, K.F. Structure dependent antioxidant capacity of phlorotannins from Icelandic Fucus vesiculosus by UHPLCDAD-ECD-QTOFMS. Food Chem. 2018, 240, 904–909.
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133.
- Gisbert, M.; Sineiro, J.; Moreira, R. Polyphenols extraction kinetics from Ascophyllum nodosum seaweed employing water and saltwater: Effect of ultrasound sonication. Algal Res. 2022, 66, 102773.
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Anal. Chem. 2015, 71, 100–109.
- Tierney, M.S.; Smyth, T.J.; Hayes, M.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Influence of pressurized liquid extraction and solid-liquid extraction methods on the phenolic content and antioxidant activities of Irish macroalgae. Int. J. Food Sci. 2013, 48, 860–869.
- Sarkar, S.; Gayen, K.; Bhowmick, T.K. Green extraction of biomolecules from algae using subcritical and supercritical fluids. Biomass Conv. Bioref. 2022, 1–23.
- Houchi, S.; Mahdadi, R.; Khenchouche, A.; Song, J.; Zhang, W.; Pang, X.; Zhang, L.; Sandalli, C.; Du, G. Investigation of common chemical components and inhibitory effect on GES-type β-lactamase (GES22) in methanolic extracts of Algerian seaweeds. Microb. Pathogenesis 2019, 126, 56–62.
- Vissers, A.M.; Caligiani, A.; Sforza, S.; Vincken, J.P.; Gruppen, H. Phlorotannin composition of Laminaria digitata. Phytochem. Anal. 2017, 28, 487–495.
- Liu, X.; Luo, G.; Wang, L.; Yuan, W. Optimization of antioxidant extraction from edible brown algae Ascophyllum nodosum using response surface methodology. Food Bioprod. Process. 2019, 114, 205–215.
- Liu, X.; Yuan, W.; Zhao, R. Extraction of antioxidants from brown algae Ascophyllum nodosum using a binary solvent extraction system. ACS Food Sci. Technol. 2021, 1, 1041–1049.
- Sánchez-Camargo, A.D.; Montero, L.; Stiger-Pouvreau, T.; Tanniou, A.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Considerations on the use of enzyme-assisted extraction in combination with pressurized liquids to recover bioactive compounds from algae. Food Chem. 2016, 192, 67–74.
- Bordoloi, A.; Goosen, N. Green and integrated processing approaches for the recovery of high-value compounds from brown seaweeds. Adv. Bot. Res. 2019, 95, 369–413.
- Tierney, M.S.; Soler-Vila, A.; Rai, D.K.; Croft, A.K.; Brunton, N.P.; Smyth, T.J. UPLC-MS profiling of low molecular weight phlorotannin polymers in Ascophyllum nodosum, Pelvetia canaliculata and Fucus spiralis. Metabolomics 2014, 10, 524–535.
- Flores-Fernandez, N.; Gonzalez-Munoz, M.J. Ultrasound-assisted extraction of bioactive carbohydrates. In Water Extraction of Bioactive Compounds; Dominguez-Gonzalez, H., Gonzalez-Munoz, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 317–331.
- Picó, Y. Ultrasound-assisted extraction for food and environmental samples. TrAC Trends Anal. Chem. 2013, 43, 84–99.
- Roselló-Soto, E.; Galanakis, C.M.; Brncic, M.; Orlien, V.; Trujillo, F.J.; Mawson, R.; Knoerzer, K.; Tiwari, B.K.; Barba, F.J. Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modelling approaches to optimize processing conditions. Trends Food Sci. Technol. 2015, 42, 134–149.
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimization of ultrasound frequency, extraction time and solvent for the recovery of polyphenols, phlorotannins and associated antioxidant activity of brown seaweeds. Mar. Drugs 2020, 18, 250.
- Sardari, R.R.; Prothmann, J.; Gregersen, O.; Turner, C.; Karlsson, E.N. Identification of phlorotannins in the brown algae, Saccharina latissima and Ascophyllum nodosum by ultra-high-performance liquid chromatography coupled to high-resolution tandem mass spectrometry. Molecules 2021, 26, 43.
- Poole, J.; Diop, A.; Rainville, L.C.; Barnabe, S. Bioextracting polyphenols from the brown seaweed Ascophyllum nodosum from Québec’s north shore coastline. Ind. Biotechnol. 2019, 15, 212–218.
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Barba, F.J.; Lorenzo, J.M. Antioxidant potential of extracts obtained from macro- (Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and micro-algae (Chlorella vulgaris and Spirulina platensis) assisted by ultrasound. Medicines 2018, 5, 33.
- Cikoš, A.M.; Jokic, S.; Šubaric, D.; Jerkovic, I. Overview on the application of modern methods for the extraction of bioactive compounds from marine macroalgae. Mar. Drugs 2018, 16, 348.
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297.
- Moreira, R.; Sineiro, J.; Chenlo, F.; Arufe, S.; Díaz-Varela, D. Aqueous extracts of Ascophyllum nodosum obtained by ultrasound-assisted extraction: Effects of drying temperature of seaweed on the properties of extracts. J. Appl. Phychol. 2017, 29, 3191–3200.
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound-assisted extraction, characterisation, and bioactivity. Mar. Drugs 2015, 13, 4270–4280.
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, S.; O’Donnell, C.P. Effect of ultrasound pretreatment on the extraction kinetics of bioactives from brown seaweed (Ascophyllum nodosum). Sep. Sci. Technol. 2015, 50, 670–675.
- Kadam, S.U.; Tiwari, B.K.; Smyth, T.J.; O’Donnell, C.P. Optimization of ultrasound assisted extraction of bioactive components from brown seaweed Ascophyllum nodosum using surface methodology. Ultrason. Sonochem. 2015, 23, 308–316.
- Pantidos, N.; Boath, A.; Lund, V.; Conner, S.; McDougall, G.J. Phenolic-rich extracts from the edible seaweed, Ascophyllum nodosum, inhibit α-amylase and α-glucosidase: Potential anti-hyperglycemic effects. J. Funct. Foods 2014, 10, 201–209.
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Grady, M.N.; Queguineur, B.; Hanni, D.; Troy, D.J.; Kerry, J.P.; O’Brien, N.M. In vitro and cellular antioxidant activities of seaweed extracts prepared from five brown seaweeds harvested in spring from the west coast of Ireland. Food Chem. 2011, 126, 1064–1070.
- Zhang, J.; Tiller, C.; Shen, J.; Wang, C.; Girouard, G.S.; Dennis, D.; Barrow, C.J.; Miao, M.; Stewart, H.S. Antidiabetic properties of polysaccharide- polyphenolic- enriched fractions from the brown seaweed Ascophyllum nodosum. Can. J. Physiol. Pharmacol. 2007, 85, 1116–1127.
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248.
- Okada, Y.; Ishimaru, A.; Suzuki, R.; Okuyama, T. A new phloroglucinol derivative from the brown alga Eisenia bicyclis: Potential for the effective treatment of diabetic complications. J. Nat. Prod. 2004, 67, 103–105.
- Jung, H.A.; Yoon, N.Y.; Woo, M.H.; Choi, J.S. Inhibitory activities of extract from several kinds of seaweeds and phlorotannins from brown alga Ecklonia stolonifera on glucose-mediated protein damage and rat lens aldose reductase. Fish Sci. 2008, 74, 1363–1365.
- Yoon, N.Y.; Lee, S.H.; Li, Y.; Kim, S.K. Phlorotannins from Ishige okamurae and acetyl- and butyrylcholinesterase inhibitory effects. J. Funct. Foods 2009, 1, 331–335.
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011, 126, 1006–1012.
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. J. Appl. Phycol. 2008, 20, 705–711.
- Nakamura, T.; Nagayama, K.; Uchida, K.; Tanaka, R. Antioxidant activity of phlorotannins isolated from the brown alga Esinia bicyclis. Fish Sci. 1996, 62, 923–926.
- Heo, S.J.; Hwang, J.Y.; Choi, J.I.; Han, J.S.; Kim, H.J.; Jeon, Y.J. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown alga, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2009, 615, 252–256.
- Fukuyama, Y.; Kodama, M.; Miura, I.; Kinzyo, Z.; Kido, M.; Mori, H.; Nakayama, Y.; Takahashi, M. Structure of an antiplasmin inhibitor, eckol, isolated from the brown algae Ecklonia kurome OKAMURA and inhibitory activities of its derivatives on plasma-plasmin inhibitor. Chem. Pharm. Bull. 1989, 37, 349–353.
- Ham, Y.M.; Baik, J.S.; Hyun, J.W.; Lee, N.H. Isolation of a new phlorotannins, fucodiphlorethol G, from a brown alga Ecklonia cava. BKCS 2007, 28, 1595–1597.
- Sailler, B.; Glombitza, K.W. Phlorethols and fucophlorethols from the brown alga Cystophora retroflexa. Phytochemistry 1999, 50, 869–881.
- Glombitza, K.W.; Keusgen, M.; Hauperich, S. Fucophlorethols from the brown algae Sargassum spinuligerum and Cystophora torulosa. Phytochemistry 1997, 46, 1417–1422.
- Fukuyama, Y.; Kodama, M.; Miura, I.; Kinzyo, Z.; Mori, H.; Nakayama, Y.; Takahashi, M. Anti-plasmin inhibitor. VI.: Structure of phlorofucofuroeckol A, a novel phlorotannin with both dibenzo-1, 4-dioxin and dibenzofuran elements, from Ecklonia kurome OKAMURA. Chem. Pharm. Bull. 1990, 38, 133–135.
- Iwai, K. Antidiabetic and antioxidant effects of polyphenols in brown alga Ecklonia stolonifera in genetically diabetic KK-Ay mice. Plant Foods Hum. Nutr. 2008, 63, 163–169.
- Sugiura, Y.; Matsuda, K.; Yamada, Y.; Nishikawa, M.; Shioya, K.; Katsumaki, H.; Imai, K.; Amano, H. Isolation of a new antiallergic phlorotannins, phlorofucofuroeckol-B, from an edible brown alga, Eiseenia arborea. Biosci. Biotechnol. Biochem. 2006, 70, 2807–2811.
- Glombitza, K.W.; Rösener, H.U. Bifuhalol: Ein diphenyläther aus Bifurcaria bifurcata. Phytochemistry 1974, 13, 1245–1247.
- Kang, K.A.; Lee, K.H.; Chae, S.W.; Koh, Y.S.; Yoo, B.S.; Kim, J.H.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Triphlorethol-A from Ecklonia cava protects V79-4 lung fibroblast against hydrogen peroxide induced cell damage. Free Radic. Res. 2005, 39, 883–892.
This entry is offline, you can click here to edit this entry!
