Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Energy & Fuels
钒氧化还原液流电池(VRFB)是大规模储能的有希望的候选者,电解质在化学-电能转换中起着关键作用。然而,由于电解质的稳定性,VRFB的工作温度被限制在10-40°C。为了克服这一点,添加了各种化学物质。
- vanadium redox flow batteries
- electrolyte additives
- electrochemical performance
- complexation
- electrostatic repulsion
- growth inhibition
- modifying electrode
1. 简介
全球能源结构正逐步从高消耗、高污染的化石燃料等不可再生能源向绿色低碳可再生能源转变。根据国家发改委22年2022月1日公布的数据,我国可再生能源装机容量已超过1亿千瓦,预计到2030年将超过煤电,成为第一大电力来源。然而,可再生能源存在不连续性、不稳定性、弃风率/光率高、电网频率/调峰困难等问题,可能无法满足不断增长的电力需求。因此,迫切需要大规模和长时间的储能技术[1,2,3,4,5]。在众多储能技术中,钒液流电池(VRFB)安全性高、循环寿命长、充放电性能好、响应速度快、容量稳定、生命周期成本低[1,6,7,8,9,10,11],是最大、技术最先进、最接近工业化的液流电池[12,13,14,15,16,17,18]。目前,全球最大的储能项目——100MW/400MWh钒电池在中国大连成功并网,连续GWh级项目已提交建设标书,标志着VRFB产业化取得重大进展。
VRFBs于1986年由Skyllas-Kazacos首次提出[19],由三个关键因素组成:电极、离子交换膜和电解质[20,21,22,23]。其中,电解液作为钒电池系统的核心部分,极大地影响了电池的能量密度和整体性能。它通常可分为正电解质和负极电解质,分别对应于公式(1)–(3)[24]中V(IV)/V(V)和V(II)/V(III)氧化还原偶的硫酸溶液。不同氧化态的相同元素可以在电极上相互转换,实现化学-电能转换,如图1所示。
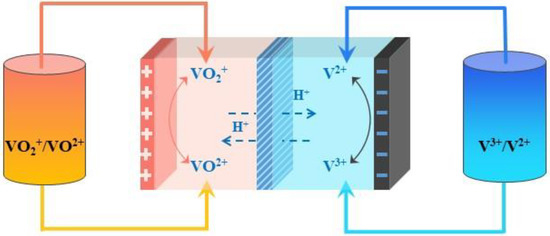
图1.钒液流电池原理图。
钒电解质一般采用物理溶解、化学还原、电解、化学-电解耦合等方法制备[25]其中,化学-电解耦合是主导方法,取高纯度V2O5作为原料并添加还原剂如H2C2O4 [26], 所以2,[27],和单质硫制取硫酸制得V(IV)电解液,再经电解还原制得V(III)电解液。如果钒电解液的工作温度高于40°C或低于10°C,钒电池的电解质稳定性和能量密度都会下降,并伴有容量损失和电池故障[28]。为了解决这个问题,在电解质[29]中添加添加剂以提高其稳定性并优化电化学动力学,从而扩大工作温度范围并提高VRFB的能量密度。例如,引入磷酸二氢铵[30]和酸性氨基酸[31]作为添加剂可以增强电解质的高温稳定性,铵和α-乳糖一水合物[32]已被用于提高电解质的低温稳定性。此外,聚丙烯酸(PAA)[33]等添加剂可以加强电化学传质。
钒电解质中的添加剂通常分为无机物、有机物和化合物,表现出不同的微观机制,包括络合、静电排斥和生长抑制。具体而言,络合通过改变电子云的分布来改善抗沉淀性能,而静电斥力通过增强V(V)离子之间的分散效应来减少V(V)离子的团聚。另一方面,生长抑制通过阻碍V的生长动力学来降低沉降颗粒的尺寸2O5.此外,钒电解质中的电化学传质增强剂主要进行亲水改性以增强电化学动力学,如MSA,可以吸附在电极上以增加活性位点。这种吸附行为可以促进界面中的氧化还原反应动力学,优化VRFB的电化学性能。因此,添加剂的引入可以有效提高钒电解液的工作温度范围,为VRFB的大规模应用提供有效的技术支持。
二、添加剂的作用机理
添加剂参与电解质性能改善的功能机制仍在研究中。由于添加剂主要分为稳定剂,包括络合剂和静电排斥剂,以及生长抑制剂和电化学增强剂,因此稳定机理和增强机理将单独讨论。电化学传质的主要稳定机理涉及络合、静电斥力和生长抑制,电化学传质的增强机理主要涉及添加剂对电极的亲水改性。
2.1. 稳定机制
2.1.1. 络合
络合是添加剂最重要的稳定机理,适用于无机和有机添加剂。如图2所示,离子(Cl−, H2采购订单4−)或官能团(-COOH,-NH2, -哦, -所以3H)携带孤对电子能够与电解质中的水合钒离子配位,与V-O-S、V-O-P、V-O-Cl、V-O-N等形成更稳定的中间体,从而有效减少V-O-V的形成,抑制V2O5降水。随后,钒的电子密度会增加,钒的局部正电荷会降低[48]。此外,形成V-O-X(X是添加剂的核心元素)的反应势垒通常低于从V形成V-O-V的反应势垒2O5,这大大降低了V的生成选择性2O5降水[49,50]。
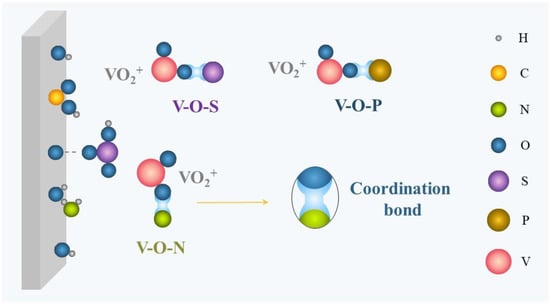
图2.络合示意图。
此外,大量研究表明,不同价态下水合钒离子的几何形状、添加剂的协同作用以及其他补充因素会影响络合行为。首先,不同价态下钒水合离子的几何形状不同,络合能力与钒离子添加剂在所有价态中的稳定性呈正相关。澄清钒离子与每种价态中的添加剂的络合对于稳定最大化和添加剂选择都是必要的。另一方面,采用协同效应可以增强稳定效果,并保持磷酸盐和铵等各种离子之间的平衡。最后,可能涉及其他补充因素,包括引入双添加剂以形成竞争关系,修改VRFB的电极,将热再生电化学循环(TREC)集成到VRFB中等。研究人员在选择添加剂时应注意上述注意事项。
取H3采购订单4例如,添加磷酸盐的电解质中V(V)的转化路径为:[VO2(H2O)2]+→ [VO(OH)2(H2O)] → VO(OH)+3 [51]. VO(OH)3中间体可以与H形成含有V-O-P键的化合物3采购订单4.而且,该反应的活化能一般低于V-O-V键的形成,可以有效避免V。2O5降水,如图3所示。此外,阴离子的主要形式是H2采购订单4−在正极电解质中加入磷酸盐添加剂后[49]。在这种情况下,由于V(V)的部分二聚化,硫酸盐以桥接或双齿配位配位与两个氧原子配位。这可以通过H的协调来解释3采购订单4或通过二聚化引起的络合模式的重排。
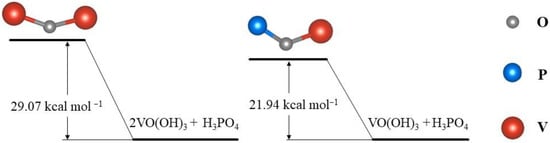
Figure 3. The energy state of the V–O–V bond and V–O–P bond.
Taking another case of Cl−, it can form the mononuclear complex VO2Cl(H2O)2 with [VO2(H2O)3]+ at high temperatures [35]. This process, with low reaction barriers and high priority, will effectively hinder the deprotonation of [VO2(H2O)3]+, serving as the first step during the precipitation reaction. Through DFT and NMR spectroscopy analyses, it is further revealed that V2O5 precipitation also could be formed by the deprotonation of di-nuclear [V2O3·8H2O]4+ cations at high temperatures, as seen in Equation (6) [48]. Nevertheless, the chlorine ion can form a stable di-nuclear complex [V2O3Cl2·6H2O]2+ with [V2O3·8H2O]4+ to prevent precipitation [48]. Figure 4 shows the geometry-optimized structures of [VO2(H2O)3]+, VO2Cl(H2O)2, [V2O3·8H2O]4+, and [V2O3Cl2·6H2O]2+. The formation of stable structures occurs because Cl2 has four groups of lone-pair electrons, which act as electron donors for complexation to the empty orbitals of vanadium ions. Furthermore, the nature of the V–O bond is the attraction of positive and negative charges; thus, the behavior of chlorine complexation makes the V–O bond weaker [48] and the O–H bond stronger, which can lead to a more stable H2O molecule and impede the deprotonation of [V2O3·8H2O]4+, thereby achieving high stability.
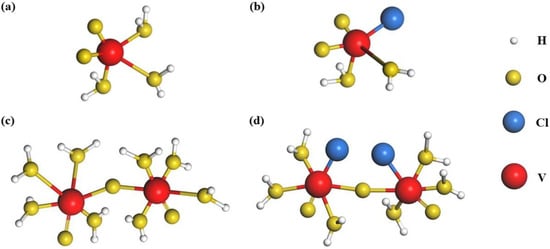
Figure 4. Geometry-optimized structures for [VO2(H2O)3]+ compound (a), chlorine bonded VO2Cl(H2O)2 compound (b), pristine di-nuclear [V2O3·8H2O]4+ compound (c), and chlorine bonded [V2O3Cl2·6H2O]2+ compound (d).
2.1.2. Electrostatic Repulsion
In electrostatic repulsion, a common stabilizing mechanism, ions or functional groups of additives, can be adsorbed on vanadium ions by electrostatic attraction, mainly including -COOH, -OH, -SO3H, -S-, -NH2, and so on. This adsorption behavior promotes the formation of ionic agglomerates with vanadium ions as the core element, which can enhance the outer layer charge and generate electrostatic repulsion to varying degrees, as shown in Figure 5. Meanwhile, due to the large core–shell spatial structure, the steric hindrance of this agglomerate encapsulating vanadium ions can strengthen this repulsion effect, making vanadium ions more dispersed, to inhibit precipitation [52]. A proliferation of studies has demonstrated the dominance of electrostatic repulsion in the stabilizing mechanism of organic additives, but the application of this principle in inorganic additives is minimal. More specifically, anionic functional groups produce negatively charged groups (e.g., -COO−) in the electrolyte, which brings about electrostatic attraction with the positively charged vanadium ions to form agglomerates. Polar groups (e.g., -NH2) tend to be adsorbed on vanadium ions based on the like-dissolves-like theory to generate external charge layers, boosting the repulsion effect [32,33,53].
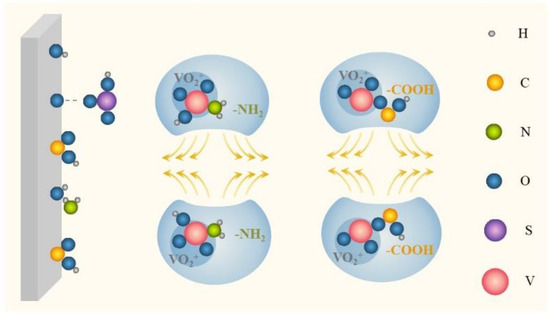
Figure 5. Schematic of electrostatic repulsion.
2.1.3. Growth Inhibition
Growth inhibition, an uncommon stabilizing mechanism, means that some additives can inhibit the growth kinetics of V2O5 precipitates in the vanadium electrolyte. As shown in Figure 6, the V2O5 precipitates can generally grow with increasing time without additives. However, after introducing some specific additives, the surfaces of the nucleation sites of the V2O5 were adsorbed by various molecules to lower the growth rate of V2O5 precipitates, lengthening the induction time of precipitation and reducing the sizes of V2O5 particles [43,52,54].
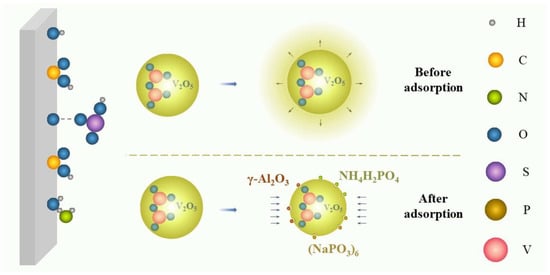
图6.生长抑制示意图。
2.2. 电化学传质增强机理
如图7所示,亥姆霍兹模型描述了由于电极-电解质界面中的相互吸引,相反的电荷如何在电解质中形成一组极板,进一步形成电容器来存储能量,称为双电层。氧化还原反应的整个过程分为反应区和转移区,其速率由电子转移和迁移传质共同控制。双电层区域的高电荷梯度降低了固液界面的传质速率,加快了反应速率。因此,在转移区,转移速率通常会减慢,从而限制了电化学性能,包括能效、容量保持率和充放电性能。因此,双电层的传质成为电化学动力学的重要调速步骤。
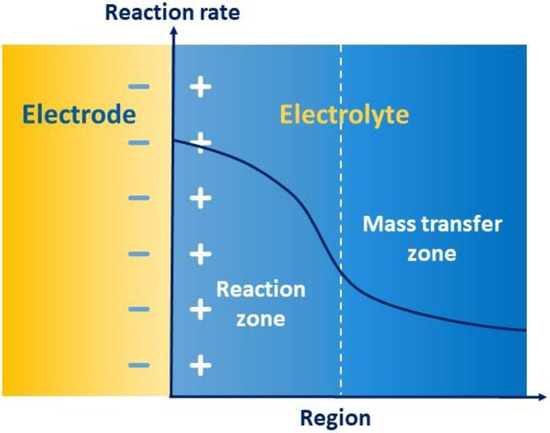
图7.双电层传质示意图。
增强电化学传质的最重要机理是添加剂可以吸附在电极表面,促进活性位点对电极形成“亲水修饰”,主要包括羟基、磺酸基、吡啶基等亲水官能团,或某些离子[32,55],如图8所示。.电极的这种改变行为可以激活电极和电解质之间的界面活性,加速所有价态中钒离子的氧化还原反应和迁移传质。或者,添加剂,如牛磺酸、MSA、PPS、过氧化苯甲酰等,可以降低VRFB的过电位,促进迁移传质,降低双电层的电阻,增强氧化还原反应的动力学[56,57,58,59,60]。
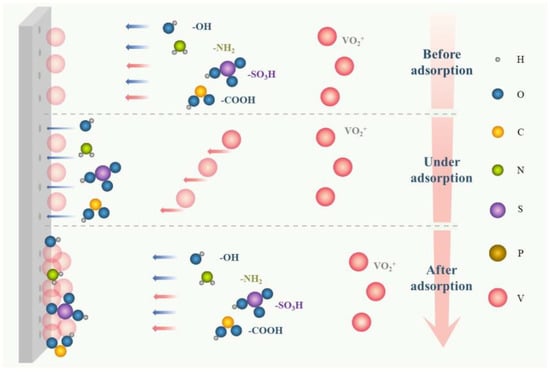
图8.电化学传质增强剂机理示意图.
This entry is adapted from the peer-reviewed paper 10.3390/ma16134582
This entry is offline, you can click here to edit this entry!
