Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Applied
A strong interest was raised in studying ceramics as potential bone grafts due to their biomechanical properties. Current biomedical applications of CaPO4-based bioceramics include artificial bone grafts, bone augmentations, maxillofacial reconstruction, spinal fusion, and periodontal disease repairs, as well as bone fillers after tumor surgery.
- biomaterials
- biomedical applications
- calcium orthophosphate
1. Introduction
One of the most exciting and rewarding areas of the engineering discipline involves development of various devices for healthcare. Some of them are implantable. Examples comprise sutures, catheters, heart valves, pacemakers, breast implants, fracture fixation plates, nails and screws in orthopedics, various filling formulations, orthodontic wires, total joint replacement prostheses, etc. However, in order to be accepted by the living body without any unwanted side effects, all implantable items must be prepared from a special class of tolerable materials, called biomedical materials or biomaterials, in short. The physical character of the majority of the available biomaterials is solids [1,2].
From the material point of view, all types of solids are divided into four major groups: metals, polymers, ceramics, and various blends thereof, called composites. Similarly, all types of solid biomaterials are also divided into the same groups: biometals, biopolymers, bioceramics, and biocomposites. All of them play very important roles in both replacement and regeneration of various human tissues; however, setting biometals, biopolymers, and biocomposites aside, this research is focused on bioceramics only. In general, bioceramics comprise various polycrystalline materials, amorphous materials (glasses), and blends thereof (glass-ceramics). Nevertheless, the chemical elements used to manufacture bioceramics form just a small set of the periodic table; namely, bioceramics might be prepared from alumina, zirconia, magnesia, carbon, silica-contained, and calcium-contained compounds, as well as from a limited number of other compounds. All these compounds might be manufactured in both dense and porous forms in bulk, as well as in the forms of crystals, powders, particles, granules, scaffolds, and/or coatings [1,2,3].
As seen from the above, the entire subject of bioceramics is still rather broad. To specify it further, let me limit myself by a description of calcium orthophosphate (abbreviated as CaPO4)-based formulations only. If compared with other types of bioceramics (such as alumina, zirconia, calcium silicates, calcium sulfate, etc.), the main feature and superiority of CaPO4 is based on their chemical similarity to the composition of calcified tissues of mammals (bones, teeth, and deer antlers) and the need for versatile and risk-free bone substitute biomaterials immediately available without the constraint of bone grafts. One of the major properties of most types of CaPO4 is their osteoconductivity, an ability to favor bone healing and to bind firmly to bone tissues. In addition, some types of CaPO4 have been shown to be able to initiate bone formation de novo in nonosseous sites [1,2,3]. Therefore, CaPO4 bioceramics are widely used in a number of different applications throughout the body, covering all areas of the skeleton. The examples include healing of bone defects, fracture treatment, total joint replacement, bone augmentation, orthopedics, cranio-maxillofacial reconstruction, spinal surgery, otolaryngology, ophthalmology, and percutaneous devices [1,2,3], as well as dental fillings and periodontal treatments [4]. Furthermore, they are also used in nonosseous applications, such as ocular implants, allowing eye movements. Depending upon the required properties, different types of CaPO4 might be used. For example, Figure 1 displays some randomly chosen samples of the commercially available CaPO4 bioceramics for bone graft applications. One should note that the global bone grafts and substitutes market was valued at USD 2.65 billion in 2020 and is projected to reach USD 3.36 billion by 2028, registering a cumulative annual growth rate of ~4.3% from 2021 to 2028 [5]. This clearly demonstrates the biomedical perspectives of CaPO4-based bioceramics.
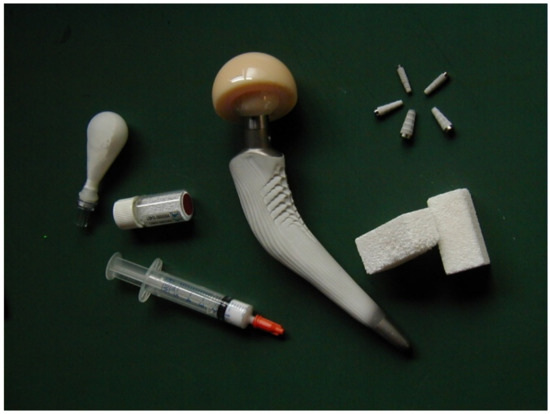
Figure 1. Several examples of the commercial CaPO4-based bioceramics.
2. Biomedical Applications
Since Levitt et al. described a method of preparing FA bioceramics and suggested their possible use in medical applications in 1969 [699], CaPO4 bioceramics have been widely tested for clinical applications. Namely, over 400 forms, compositions, and trademarks (Table 1) are currently either in use or under consideration in many areas of orthopedics and dentistry [700], with even more in development. In addition, various formulations containing demineralized bone matrix (commonly abbreviated as DBM) are produced for bone grafting. For example, bulk materials, available in dense and porous forms, are used for alveolar ridge augmentation, immediate tooth replacement, and maxillofacial reconstruction [4,701]. Other examples comprise burr-hole buttons [702,703], cosmetic (nonfunctional) eye replacements such as Bio-Eye® [704,705,706,707,708,709], increment of the hearing ossicles [710,711,712], and spine fusion [713,714,715,716], as well as repair of bone [118,717,718], craniofacial [719], and dental [720] defects. In order to permit growth of new bone into defects, a suitable bioresorbable material should fill these defects. Otherwise, ingrowth of fibrous tissue might prevent bone formation within the defects.
Table 1. Registered commercial trademarks (current and past) of CaPO4-based bioceramics and biomaterials.
| Calcium Orthophosphate | Trade Name and Producer (When Available) |
|---|---|
| CDHA | Calcibon (Zimmer Biomet, IN, USA) |
| Cementek (Teknimed, France) | |
| CHT Ceramic Hydroxyapatite (Bio-Rad, CA, USA) | |
| nanoXIM (Fluidinova, Portugal) | |
| OsteoGen (Impladent, NY, USA) | |
| without trade name (Himed, NY, USA) | |
| HA | Actifuse (ApaTech, UK) |
| Alveograf (Cooke-Waite Laboratories, USA) | |
| Apaceram (HOYA Technosurgical, Japan) | |
| Apafill-G (Habana, Cuba) | |
| ApaPore (ApaTech, UK) | |
| BABI-HAP (Berkeley Advanced Biomaterials, CA, USA) | |
| Bio-Eye (Integrated Orbital Implants, CA, USA) | |
| BIOGAP (Connectbiopharm, Russia) | |
| BioGraft (IFGL BIO CERAMICS, India) | |
| Bioroc (Depuy Bioland, France) | |
| Blue Bone (Regener Biomateriais, Brazil) | |
| Boneceram (Sumitomo Osaka Cement, Japan) | |
| Bonefil (Pentax, Japan) | |
| BoneSource (Stryker Orthopaedics, NJ, USA) | |
| Bonetite (Pentax, Japan) | |
| Bonfil (Mitsubishi Materials, Japan) | |
| Bongros-HA (Daewoong Pharmaceutical, Korea) | |
| CAFOS DT (Chemische Fabrik Budenheim, Germany) | |
| Calcitite (Sulzer Calcitek, CA, USA) | |
| CAMCERAM HA (CAM Implants, Netherlands) | |
| CAPTAL (Plasma Biotal, UK) | |
| CELLYARD (HOYA Technosurgical, Japan) | |
| Cerapatite (Ceraver, France) | |
| Ceros HA (Mathys, Switzerland) | |
| CHT Ceramic Hydroxyapatite (Bio-Rad, CA, USA) | |
| Durapatite (unknown producer) | |
| ENGIpore (JRI Orthopaedics, UK) | |
| G-Bone (Surgiwear, India) | |
| GranuMas (GranuLab, Malaysia) | |
| HA BIOCER (CHEMA – ELEKTROMET, Poland) | |
| HAnano Surface (Promimic, Sweden) | |
| HAP-91 (JHS Biomateriais, Brazil) | |
| HAP-99 (Polystom, Russia) | |
| HAP–Bionnovation (Bionnovation, Brazil) | |
| IngeniOs HA (Zimmer Dental, CA, USA) | |
| Micro Crystalline Hydroxyapatite Complex (MCHC) (Clarion Pharmaceutical, India) | |
| nanoXIM (Fluidinova, Portugal) | |
| Neobone (Covalent Materials, Japan) | |
| Osbone (Curasan, Germany) | |
| OsproLife HA (Lincotek Medical, Italy) | |
| Ossein Hydroxyapatite (Clarion Pharmaceutical, India) | |
| OssaBase-HA (Lasak, Czech Republic) | |
| Ostegraf (Ceramed, CO, USA) | |
| Ostim (Heraeus Kulzer, Germany) | |
| Ovis Bone HA (DENTIS, Korea) | |
| Periograf (Cooke-Waite Laboratories, USA) | |
| PermaOS (Mathys, Switzerland) | |
| PRINT3D Hydroxyapatite (Prodways, France) | |
| Pro Osteon (Zimmer Biomet, IN, USA) | |
| PurAtite (PremierBiomaterials, Ireland) | |
| REGENOS (Kuraray, Japan) | |
| SHAp (SofSera, Japan) | |
| Synatite (SBM, France) | |
| Synthacer (KARL STORZ Recon, Germany) | |
| Theriridge (Therics, OH, USA) | |
| without trade name (Cam Bioceramics, Netherlands) | |
| without trade name (CaP Biomaterials, WI, USA) | |
| without trade name (DinganTec, China) | |
| without trade name (Ensail Beijing, China) | |
| without trade name (Himed, NY, USA) | |
| without trade name (MedicalGroup, France) | |
| without trade name (SANGI, Japan) | |
| without trade name (Shanghai Rebone Biomaterials, China) | |
| without trade name (SigmaGraft, CA, USA) | |
| without trade name (SkySpring Nanomaterials, TX, USA) | |
| without trade name (SofSera, Japan) | |
| without trade name (Taihei Chemical Industrial, Japan) | |
| without trade name (Xpand Biotechnology, Netherlands) | |
| Mg-HA | SINTlife (JRI Orthopaedics, UK) |
| HA powder suspended in water | Ostibone (FH Orthopedics, France) |
| NANOSTIM (Medtronic Sofamor Danek, TN, USA) | |
| n-IBS (Bioceramed, Portugal) | |
| Skelifil (Osteotec, UK) | |
| HA embedded or suspended in a gel | Bio-Gel HT hydroxyapatite (Bio-Rad, CA, USA) |
| Coaptite (Boston Scientific, MA, USA) | |
| Facetem (Daewoong, Korea) | |
| NanoBone (Artoss, Germany) | |
| Nanogel (Teknimed, France) | |
| Radiesse (Merz Aesthetics, Germany) | |
| Renú Calcium Hydroxylapatite Implant (Cytophil, WI, USA) | |
| HA/collagen, CDHA/collagen and/or carbonate apatite/collagen | AUGMATRIX (Wright Medical Technology, TN, USA) |
| Bioimplant (Connectbiopharm, Russia) | |
| Bio-Oss Collagen (Geitslich, Switzerland) | |
| Boneject (Koken, Japan) | |
| COL.HAP-91 (JHS Biomateriais, Brazil) | |
| Collagraft (Zimmer and Collagen Corporation, USA) | |
| CollaOss (SK Bioland, Korea) | |
| CollapAn (Intermedapatite, Russia) | |
| COLLAPAT (Symatese, France) | |
| DualPor collagen (OssGen, Korea) | |
| G-Graft (Surgiwear, India) | |
| HAPCOL (Polystom, Russia) | |
| Healos (DePuy Spine, USA) | |
| LitAr (LitAr, Russia) | |
| Ossbone Collagen (SK Bioland, Korea) | |
| OssFill (Sewon Cellontech, Korea) | |
| OssiMend (Collagen Matrix, NJ, USA) | |
| Osteomatrix (Connectbiopharm, Russia) | |
| OsteoTape (Impladent, NY, USA) | |
| ReFit (HOYA Technosurgical, Japan | |
| RegenOss (JRI Orthopaedics, UK) | |
| RegenerOss Synthetic (Zimmer Dental, CA, USA) | |
| Straumann XenoFlex (Straumann, Switzerland) | |
| HA/sodium alginate | Bialgin (Biomed, Russia) |
| HA/poly-L-lactic acid | Biosteon (Biocomposites, UK) |
| ReOss (ReOss, Germany) | |
| OSTEOTRANS MX (Teijin Medical Technologies, Japan) | |
| SuperFIXSORB30 (Takiron, Japan) | |
| HA/polyethylene | HAPEX (Gyrus, TN, USA) |
| HA/CaSO4 | BioWrist Bone Void Filler (Skeletal Kinetics, CA, USA) |
| Bond Apatite (Augma Biomaterials, NJ, USA) | |
| Hapset (LifeCore, MN, USA) | |
| PerOssal (aap Implantate, Germany) | |
| HA/CaSO4 powders suspended in a liquid | CERAMENT (BONESUPPORT, Sweden) |
| Coralline HA | Biocoral (Bio Coral Calcium Bone, France) |
| BoneMedik-S (Meta Biomed, Korea) | |
| Interpore (Interpore, CA, USA) | |
| ProOsteon (Interpore, CA, USA) | |
| Carbonate apatite | Cytrans (GC, Japan) |
| Norian SRS (Norian, CA, USA) | |
| Algae-derived HA | Algipore (AlgOss Biotechnologies, Austria) |
| Algisorb (AlgOss Biotechnologies, Austria) | |
| FRIOS Algipore (DENTSPLY Implants, Sweden) | |
| SIC nature graft (AlgOss Biotechnologies, Austria) | |
| HA/glass | Bonelike (unknwn producer) |
| Bovine bone (unsintered) | Unilab Surgibone (Unilab, NJ, USA) |
| Bovine bone (unsintered) + polymer | Alpha-Bio’s Graft (Alpha-Bio Tec, Israel) |
| C-Graft Putty (unknwn producer) | |
| Bovine bone apatite (unsintered) | Apatos (OsteoBiol, Italy) |
| Bio-Oss (Geistlich Biomaterials, Switzerland) | |
| Bonefill (Bionnovation, Brazil). | |
| CANCELLO-PURE (Wright Medical Technology, TN, USA) | |
| CenoBone (Tissue Regeneration Corporation, Iran) | |
| CopiOs Cancellous Particulate Xenograft (Zimmer, IN, USA) | |
| GenOs (OsteoBiol, Italy) | |
| InterOss (SigmaGraft, CA, USA) | |
| Laddec (Ost-Developpement, France) | |
| Lubboc (Ost-Developpement, France) | |
| MatrixCellect (Curasan, Germany) | |
| Mega-Oss Bovine (Megagen Implant, Korea) | |
| Orthoss (Geitslich, Switzerland) | |
| OssiGuide (Collagen Matrix, NJ, USA) | |
| Oxbone (Bioland biomateriaux, France) | |
| Straumann XenoGraft (Straumann, Switzerland) | |
| Surgibone (Surgibon, Ecuador) | |
| Tutobone (Tutogen Medical, Germany) | |
| Tutofix (Tutogen Medical, Germany) | |
| Tutoplast (Tutogen Medical, Germany) | |
| without trade name (MedicalGroup, France) | |
| Porcine bone apatite (unsintered) | A-OSS (Osstem Implant, Korea) |
| GEM Bone Graft (Lynch Biologics, USA) | |
| Gen-Os (OsteoBiol, Italy) | |
| MatrixOss (Collagen Matrix, NJ, USA) | |
| OsteoBiol (OsteoBiol, Italy) | |
| Symbios Xenograft (DENTSPLY Implants, Sweden) | |
| THE Graft (Purgo Biologics, Korea) | |
| Equine bone apatite (unsintered) | BIO-GEN (BioTECK, Italy) |
| Sp-Block (OsteoBiol, Italy) | |
| Bovine bone apatite (sintered) | 4Bone XBM (MIS Implants, Israel) |
| BonAP (unknown producer) | |
| Cerabone (aap Implantate, Germany and botiss, Germany) | |
| Endobon (Merck, Germany) | |
| GenoxInorgânico (Baumer, SP, Brazil) | |
| Iceberg oss (Global Medical Implants, Spain) | |
| Navigraft (Zimmer Dental, USA) | |
| Osteograf (Ceramed, CO, USA) | |
| OVIS XENO (DENTIS, Korea) | |
| PepGen P-15 (DENTSPLY Implants, Sweden) | |
| Pyrost (Osteo AG, Germany) | |
| Sinbone (Purzer Pharmaceutical, Taiwan) | |
| SynOss (Collagen Matrix, NJ, USA) | |
| Straumann cerabone (Straumann, Switzerland) | |
| Human bone allograft | ALLOPURE (Wright Medical Technology, TN, USA) |
| Allosorb (Curasan, Germany) | |
| CancellOss (Impladent, NY, USA) | |
| CurOss (Impladent, NY, USA) | |
| J Bone Block (Impladent, NY, USA) | |
| maxgraft (botiss, Germany) | |
| Mega-Oss (Megagen Implant, Korea) | |
| NonDemin (Impladent, NY, USA) | |
| Osnatal (aap Implantate, Germany) | |
| OsteoDemin (Impladent, NY, USA) | |
| OsteoWrap (Curasan, Germany) | |
| OVIS ALLO (DENTIS, Korea) | |
| PentOS OI (Citagenix, QC, Canada) | |
| RAPTOS (Citagenix, QC, Canada) | |
| Straumann AlloGraft (Straumann, Switzerland) | |
| TenFUSE (Wright Medical Technology, TN, USA) | |
| α-TCP | BioBase (Biovision, Germany) |
| Tetrabone (unknown producer) | |
| without trade name (Cam Bioceramics, Netherlands) | |
| without trade name (DinganTec, China) | |
| without trade name (Ensail Beijing, China) | |
| without trade name (Himed, NY, USA) | |
| without trade name (InnoTERE, Germany) | |
| without trade name (PremierBiomaterials, Ireland) | |
| without trade name (Taihei Chemical Industrial, Japan) | |
| β-TCP | AdboneTCP (Medbone Medical Devices, Portugal) |
| AFFINOS (Kuraray, Japan) | |
| Allogran-R (Biocomposites, UK) | |
| Antartik TCP (MedicalBiomat, France) | |
| ArrowBone (Brain Base Corporation, Japan) | |
| AttraX scaffold (NuVasive, CA, USA) | |
| BABI-TCP (Berkeley Advanced Biomaterials, CA, USA) | |
| Betabase (Biovision, Germany) | |
| BioGraft (IFGL BIO CERAMICS, India) | |
| Bioresorb (Sybron Implant Solutions, Germany) | |
| Biosorb (SBM, France) | |
| Bi-Ostetic (Berkeley Advanced Biomaterials, CA, USA) | |
| Bonegraft (Bonegraft biomaterials, Turkey) | |
| BoneSigma TCP (SigmaGraft, CA, USA) | |
| C 13-09 (Chemische Fabrik Budenheim, Germany) | |
| Calc-i-oss classic (Degradable Solutions, Switzerland) | |
| Calciresorb (Ceraver, France) | |
| CAMCERAM TCP (CAM Implants, Netherlands) | |
| CAPTAL β-TCP (Plasma Biotal, UK) | |
| CELLPLEX (Wright Medical Technology, TN, USA) | |
| Cerasorb (Curasan, Germany) | |
| Ceros TCP (Mathys, Switzerland) | |
| ChronOS (Synthes, PA, USA) | |
| Cidemarec (KERAMAT, Spain) | |
| Conduit (DePuy Spine, USA) | |
| cyclOS (Mathys, Switzerland) | |
| ExcelOs (BioAlpha, Korea) | |
| GenerOs (Berkeley Advanced Biomaterials, CA, USA) | |
| HT BIOCER (CHEMA – ELEKTROMET, Poland) | |
| Iceberg TCP (Global Medical Implants, Spain) | |
| IngeniOs β-TCP (Zimmer Dental, CA, USA) | |
| ISIOS+ (Kasios, France) | |
| JAX (Smith and Nephew Orthopaedics, USA) | |
| Keramedic (Keramat, Spain) | |
| KeraOs (Keramat, Spain) | |
| Mega-TCP (Megagen Implant, Korea) | |
| microTCP (Conmed, USA) | |
| nanoXIM (Fluidinova, Portugal) | |
| Orthograft (DePuy Spine, USA) | |
| Ossaplast (Ossacur, Germany) | |
| Osferion (Olympus Terumo Biomaterials, Japan) | |
| Osfill (Olympus Terumo Biomaterials, Japan) | |
| OsproLife β-TCP (Lincotek Medical, Italy) | |
| OsSatura TCP (Integra Orthobiologics, CA, USA) | |
| Ossoconduct (SteinerBio, NV, USA) | |
| Osteoblast (Galimplant, Spain) | |
| Osteocera (Hannox, Taiwan) | |
| Osteopore TCP (SpiteCraft, IL, USA) | |
| OSTEOwelt (Biolot Medical, Turkey) | |
| Periophil β-TCP (Cytophil, WI, USA) | |
| Platon Pearl Bone (Platon, Japan) | |
| PolyBone (Kyungwon Medical, Korea) | |
| PORESORB-TCP (Lasak, Czech Republic) | |
| Powerbone (Medical Expo Bonegraft Biomaterials, Spain) | |
| PRINT3D Tricalcium Phosphate (Prodways, France) | |
| Repros (JRI Orthopaedics, UK) | |
| R.T.R. (Septodont, PA, USA) | |
| SigmaOs TCP (SigmaGraft, CA, USA) | |
| Socket Graft (SteinerBio, NV, USA) | |
| Sorbone (Meta Biomed, Korea) | |
| SUPERPORE (HOYA Technosurgical, Japan) | |
| Suprabone TCP (BMT Group, Turkey) | |
| Syncera (Oscotec, Korea) | |
| SynthoGraft (Bicon, MA, USA) | |
| Synthos (unknown producer) | |
| Syntricer (KARL STORZ Recon, Germany) | |
| TCP (Kasios, France) | |
| Terufill (Olympus Terumo Biomaterials, Japan) | |
| TKF-95 (Polystom, Russia) | |
| TriCaFor (BioNova, Russia) | |
| Triha+ (Teknimed, France) | |
| TriOSS (Bioceramed, Portugal) | |
| Vitomatrix (Orthovita, PA, USA) | |
| Vitoss (Orthovita, PA, USA) | |
| without trade name (CaP Biomaterials, WI, USA) | |
| without trade name (Cam Bioceramics, Netherlands) | |
| without trade name (DinganTec, China) | |
| without trade name (Ensail Beijing, China) | |
| without trade name (Himed, NY, USA) | |
| without trade name (Shanghai Bio-lu Biomaterials, China) | |
| without trade name (Shanghai Rebone Biomaterials, China) | |
| without trade name (SigmaGraft, CA, USA) | |
| without trade name (Taihei Chemical Industrial, Japan) | |
| without trade name (Xpand Biotechnology, Netherlands) | |
| β-TCP/CaSO4 | Fortoss vital (Biocomposites, UK) |
| Genex (Biocomposites, UK) | |
| β-TCP/poly-lactic acid | Bilok (Biocomposites, UK) |
| Duosorb (SBM, France) | |
| Matryx Interference Screws (Conmed, USA) | |
| β-TCP/poly-lactic-co-glycolic acid | Evolvemer TCP30PLGA (Arctic Biomaterials, Finland) |
| β-TCP/polymer | AttraX putty (NuVasive, CA, USA) |
| Therigraft (Therics, OH, USA) | |
| β-TCP/bone marrow aspirate | Induce (Skeletal Kinetics, CA, USA) |
| β-TCP/collagen | Integra Mozaik (Integra Orthobiologics, CA, USA) |
| β-TCP/growth-factor | GEM 21S (Lynch Biologics, USA) |
| β-TCP/rhPDGF-BB solution | AUGMENT Bone Graft (Wright Medical Group, TN, USA) |
| BCP (HA + β-TCP) | 4Bone BCH (MIS Implants, Israel) |
| adboneBCP (Medbone Medical Devices, Portugal) | |
| Antartik (MedicalBiomat, France) | |
| ARCA BONE (ARCA-MEDICA, Switzerland) | |
| Artosal (aap Implantate, Germany) | |
| BABI-HATCP (Berkeley Advanced Biomaterials, CA, USA) | |
| Bicera (Hannox, Taiwan) | |
| BCP BiCalPhos (Medtronic, MN, USA) | |
| BIO-C (Cowellmedi, Korea) | |
| BioActys (Graftys, France) | |
| BioGraft (IFGL BIO CERAMICS, India) | |
| Biosel (Depuy Bioland, France) | |
| BonaGraft (Biotech One, Taiwan) | |
| Boncel-Os (BioAlpha, Korea) | |
| Bone Plus BCP (Megagen Implant, Korea) | |
| Bone Plus BCP Eagle Eye (Megagen Implant, Korea) | |
| BoneMedik-DM (Meta Biomed, Korea) | |
| BoneSave (Stryker Orthopaedics, NJ, USA) | |
| BoneSigma BCP (SigmaGraft, CA, USA) | |
| BONITmatrix (DOT, Germany) | |
| Calcicoat (Zimmer, IN, USA) | |
| Calciresorb (Ceraver, France) | |
| Calc-i-oss crystal (Degradable Solutions, Switzerland) | |
| CellCeram (Scaffdex, Finland) | |
| Ceraform (Teknimed, France) | |
| Ceratite (NGK Spark Plug, Japan) | |
| Cross.Bone (Biotech Dental, France) | |
| CuriOs (Progentix Orthobiology BV, Netherlands) | |
| DM-Bone (Meta Biomed, Korea) | |
| Eclipse (Citagenix, QC, Canada) | |
| Eurocer (FH Orthopedics, France) | |
| Frabone (Inobone, Korea) | |
| Genesis-BCP (DIO, Korea) | |
| GenPhos HA TCP (Baumer, Brazil) | |
| Graftys BCP (Graftys, France) | |
| Hatric (Arthrex, Naples, FL, USA) | |
| Hydroxyapol (Polystom, Russia) | |
| Kainos (Signus, Germany) | |
| MagnetOs (Kuros Biosciences, Switzerland) | |
| MasterGraft (Medtronic Sofamor Danek, TN, USA) | |
| Maxresorb (botiss, Germany) | |
| MBCP (Biomatlante, France) | |
| MimetikOss (Mimetis Biomaterials, Spain) | |
| Neobone (Bioceramed, Portugal) | |
| New Bone (GENOSS, Korea) | |
| NT-BCP (OssGen, Korea) | |
| NT-Ceram (Meta Biomed, Korea) | |
| OdonCer (Teknimed, France) | |
| OpteMX (Exactech, FL, USA) | |
| OrthoCer HA TCP (Baumer, Brazil) | |
| OsproLife HA-βTCP (Lincotek Medical, Italy) | |
| OsSatura BCP (Integra Orthobiologics, CA, USA) | |
| ossceram nano (bredent medical, Germany) | |
| OSSEOPLUS (JHS Biomateriais, Brazil) | |
| Osspol (Genewel, Korea) | |
| OsteoFlux (VIVOS-Dental, Switzerland) | |
| Osteon (GENOSS, Korea) | |
| Osteosynt (Einco, Brazil) | |
| Ostilit (Stryker Orthopaedics, NJ, USA) | |
| Ovis Bone BCP (DENTIS, Korea) | |
| Periophil biphasic (Cytophil, WI, USA) | |
| Q-OSS+ (Osstem Implant, Korea) | |
| ReproBone (Ceramisys, UK) | |
| R.T.R.+ (Septodont, PA, USA) | |
| SBS (Expanscience, France) | |
| Scaffdex (Scaffdex Oy, Finland) | |
| SigmaOs BCP (SigmaGraft, CA, USA) | |
| SinboneHT (Purzer Pharmaceutical, Taiwan) | |
| SkeliGraft (Osteotec, UK) | |
| Straumann BoneCeramic (Straumann, Switzerland) | |
| SYMBIOS Biphasic Bone Graft Material (DENTSPLY Implants, Sweden) | |
| SynMax (BioHorizons, Spain) | |
| Synergy (unknown producer) | |
| TCH (Kasios, France) | |
| Topgen-S (Toplan, Korea) | |
| Tribone (Stryker, Europe) | |
| Triosite (Zimmer, IN, USA) | |
| without trade name (AlgOss Biotechnologies, Austria) | |
| without trade name (Cam Bioceramics, Netherlands) | |
| without trade name (CaP Biomaterials, WI, USA) | |
| without trade name (Himed, NY, USA) | |
| without trade name (MedicalGroup, France) | |
| without trade name (SigmaGraft, CA, USA) | |
| without trade name (Xpand Biotechnology, Netherlands) | |
| BCP (HA + α-TCP) | Skelite (Millennium Biologix, ON, Canada) |
| BCP (HA + β-TCP)/collagen | Allograft (Zimmer, IN, USA) |
| collacone max (botiss, Germany) | |
| Collagraft (Zimmer, IN, USA) | |
| Cross.Bone Matrix (Biotech Dental, France) | |
| Indost (Polystom, Russia) | |
| MasterGraft (Medtronic Sofamor Danek, TN, USA) | |
| MATRI BONE (Biom’Up, France) | |
| Osteon III collagen (GENOSS, Korea) | |
| SynergOss (Nobil Bio Ricerche, Italy) | |
| without trade name (MedicalGroup, France) | |
| BCP (HA + β-TCP)/hydrogel | 4MATRIX+ (MIS Implants, Israel) |
| Eclipse (Citagenix, QC, Canada) | |
| BCP (HA + β-TCP)/polymer | In’Oss (Biomatlante, France) |
| Hydros (Biomatlante, France) | |
| Osteocaf (Texas Innovative Medical Devices, TX, USA) | |
| Osteotwin (Biomatlante, France) | |
| BCP (HA + TTCP) | OsproLife HA-TTCP (Lincotek Medical, Italy) |
| BCP (HA + β-TCP)/chitosan | k-IBS (Bioceramed, Portugal) |
| BCP (HA + β-TCP)/fibrin | TricOS (Baxter BioScience, France) |
| BCP (HA + β-TCP)/silicon | FlexHA (Xomed, FL, USA) |
| Bioglass + α-TCP + β-TCP + HA + polymers | OsteoFlo NanoPutty (SurGenTec, FL, USA) |
| FA | without trade name (CaP Biomaterials, WI, USA) |
| FA + BCP (HA + β-TCP) | FtAP (Polystom, Russia) |
| DCPA | without trade name (Himed, NY, USA) |
| without trade name (Shanghai Rebone Biomaterials, China) | |
| DCPA + MgHPO4·3H2O + SiO2 + carboxymethyl cellulose | Novogro (OsteoNovus, OH, USA) |
| DCPD | without trade name (Himed, NY, USA) |
| DCPD/collagen | CopiOs Bone Void Filler (Zimmer, IN, USA) |
| DCPD + β-TCP/CaSO4 | PRO-DENSE (Wright Medical Group, TN, USA) |
| DCPD + β-TCP/CaSO4 + collagen | PRO-STIM (Wright Medical Group, TN, USA) |
| ACP | CAPTAL ACP (Plasma Biotal, UK) |
| without trade name (Himed, NY, USA) | |
| OCP | Bontree (HudensBio, Korea) |
| OctoFor (BioNova, Russia) | |
| without trade name (Himed, NY, USA) | |
| OCP/fibrin | FibroFor (BioNova, Russia) |
| OCP/collagen | Bonarc (Toyobo, Japan) |
| TTCP | without trade name (Ensail Beijing, China) |
| without trade name (Himed, NY, USA) | |
| without trade name (Shanghai Rebone Biomaterials, China) | |
| without trade name (Taihei Chemical Industrial, Japan) | |
| Undisclosed CaPO4 | Arex Bone (Osteotec, UK) |
| Inno-CaP (Cowellmedi, Korea) | |
| Undisclosed CaPO4 + biologics | i-FACTOR (Cerapedics, CO, USA) |
| MCPM | Phosfeed MCP (OCP group, Morocco) |
| MCPM + DCPD | Phosfeed MDCP (OCP group, Morocco) |
In spite of the aforementioned serious mechanical limitations (see Mechanical Properties), bioceramics of CaPO4 are available in various physical forms: powders, particles, granules (or granulates), dense blocks, porous scaffolds, self-setting formulations, implant coatings, and composite components of different origin (natural, biological, or synthetic), often with specific shapes, such as implants, prostheses, or prosthetic devices. In addition, CaPO4 are also applied as nonhardening injectable formulations [721,722,723,724,725,726] and pastes [726,727,728,729,730]. Generally, they consist of a mixture of CaPO4 powders or granules and a “glue”, which can be a highly viscous hydrogel. More to the point, custom-designed shapes such as wedges for tibial opening osteotomy, cones for spine and knee, and inserts for vertebral cage fusion are also available [546]. Various trademarks of the commercially available types of CaPO4-based bioceramics and biomaterials are summarized in Table 1, while their surgical applications are schematically shown in [731]. A long list of both trademarks and producers clearly demonstrates that CaPO4 bioceramics are easy to make and not very difficult to register for biomedical applications. There is an ISO standard for CaPO4-based bone substitutes [732].
One should note that among the existing CaPO4, only certain compounds are useful for biomedical applications, because those having a Ca/P ionic ratio less than 1 are not suitable for implantation due to their high solubility and acidity. Furthermore, due to its basicity, TTCP alone cannot be suitable either. Nevertheless, researchers try [141]. In addition, to simplify biomedical applications, these “of little use” CaPO4 can be successfully combined with either other types of CaPO4 or other chemicals.
2.1. Self-Setting (Self-Hardening) Formulations
The need for bioceramics for minimal invasive surgery has induced the concept of self-setting (or self-hardening) formulations consisting of CaPO4 only to be applied as injectable and/or moldable bone substitutes [102,103,124,507,733]. After hardening, they form bulk CaPO4 bioceramics. In addition, there are reinforced formulations that, in a certain sense, might be defined as CaPO4 concretes [102]. Furthermore, self-setting formulations able to produce porous bulk CaPO4 bioceramics are also available [450,451,458,459,460,461,507,520,733,734,735,736].
All types of the self-setting CaPO4 formulations belong to low-temperature bioceramics. They are divided into two major groups. The first one is a dry mixture of two different types of CaPO4 (a basic one and an acidic one), in which, after being wetted, the setting reaction occurs according to an acid–base reaction. The second group contains only one CaPO4, such as ACP with Ca/P molar ratio within 1.50–1.67 or α-TCP: both of them form CDHA upon contact with an aqueous solution [102,124]. Chemically, setting (= hardening, curing) is due to the succession of dissolution and precipitation reactions. Mechanically, it results from crystal entanglement and intergrowth [737]. By influencing dimensions of forming CaPO4 crystals, it is possible to influence the mechanical properties of the hardened bulk bioceramics [738]. Sometimes, the self-set formulations are sintered to prepare high-temperature CaPO4 bioceramics [739]. Despite a large number of initial compositions, all types of self-setting CaPO4 formulations can form three products only: CDHA, DCPD, and, rarely, DCPA [102,103,124,507,733]. Special reviews on the topic are available in [102,103,739], where interested readers are referred for further details.
2.2. CaPO4 Deposits (Coatings, Films, and Layers)
For many years, the clinical application of CaPO4-based bioceramics has been largely limited to non-load-bearing parts of the skeleton due to their inferior mechanical properties. Therefore, materials with better mechanical properties appear to be necessary. For example, metallic implants are encountered in endoprostheses (total hip joint replacements) and artificial teeth sockets. As metals do not undergo bone bonding, i.e., they do not form a mechanically stable link between the implant and bone tissue, methods have been sought to improve contacts at the interface. One major method is to coat metals with CaPO4, which enables bonding ability between the metal and the bone [180,190,397,740,741,742].
A number of factors influence the properties of CaPO4 deposits (coatings, films, and layers). They include thickness (this will influence coating adhesion and fixation—the agreed optimum now seems to be within 50–100 µm), crystallinity (this affects the dissolution and biological behavior), phase and chemical purity, porosity, and adhesion. The coated implants combine the surface biocompatibility and bioactivity of CaPO4 with the core strength of strong substrates. Moreover, CaPO4 deposits decrease a release of potentially hazardous chemicals from the core implant and shield the substrate surface from environmental attack. In the case of porous implants, the CaPO4-coated surface enhances bone ingrowth into the pores [331]. The clinical results for CaPO4-deposited implants reveal that they have much longer lifetimes after implantation than uncoated devices and they are found to be particularly beneficial for younger patients. Further details on this topic are available in the special reviews [740,741,742].
2.3. Functionally Graded Bioceramics
In general, functionally gradient materials (FGMs) are defined as materials having either compositional or structural gradient from their surface to the interior. The idea of FGMs allows one device to possess two different properties. One of the most important combinations for the biomedical field is that of mechanical strength and biocompatibility. Namely, only surface properties govern a biocompatibility of the entire device. In contrast, the strongest material determines the mechanical strength of the entire device. Although this subject belongs to the previous section on coatings, films, and layers, in a certain sense, all types of implants covered by CaPO4 might be also considered as FGMs.
Within the scope of this research, functionally graded bioceramics consisting of CaPO4 are considered and discussed only. Such formulations have been developed [74,491,494,550,743,744,745,746,747,748,749,750,751,752,753]. For example, dense sintered bodies with gradual compositional changes from α-TCP to HA were prepared by sintering diamond-coated HA compacts at 1280 °C under a reduced pressure, followed by heating under atmospheric conditions [743]. The content of α-TCP gradually decreased, while the content of HA increased with increasing depth from the surface. This functionally gradient bioceramic consisting of HA core and α-TCP surface showed potential value as a bone-substituting biomaterial [743]. Two types of functionally gradient FA/β-TCP biocomposites were prepared in another study [744]. One of the graded biocomposites was in the shape of a disk and contained four different layers of about 1 mm thick. The other graded biocomposite was also in the shape of a disk but contained two sets of the four layers, each layer being 0.5 mm thick controlled by using a certain amount of the mixed powders. The final FA/β-TCP graded structures were formed at 100 MPa and sintered at 1300 °C for 2 h [744]. The same approach was used in yet another study, but HA was used instead of FA and CDHA was used instead of β-TCP [752]. CaPO4 coatings with graded crystallinity were prepared as well [748].
In addition, it is well known that a bone cross-section from cancellous to cortical bone is nonuniform in porosity and pore dimensions. Thus, in various attempts to mimic the porous structure of bones, CaPO4 bioceramics with graded porosity have been fabricated [74,432,478,491,494,550,743,744,745,746]. For example, graded porous CaPO4 bioceramics can be produced by means of tape casting and lamination. Other manufacturing techniques, such as a compression molding process followed by impregnation and firing, are known as well [432]. In the first method, an HA slurry was mixed with a pore former. The mixed slurry was then cast into a tape. Using the same method, different tapes with different pore former sizes were prepared individually. The different tape layers were then laminated together. Firing was then performed to remove the pore formers and sinter the HA particle compacts, resulting in graded porous bioceramics [746]. This method was also used to prepare graded porous HA with a dense part (core or layer) in order to improve the mechanical strength, as dense ceramics are much stronger than porous ceramics. However, as in the pressure infiltration of mixed particles, this multiple tape casting also has the problem of poor connectivity of pores, although the pore size and the porosity are relatively easy to control. Furthermore, the lamination step also introduces additional discontinuity of the porosity on the interfaces between the stacked layers.
Since diverse biomedical applications require different configurations and shapes, the graded (or gradient) porous bioceramics can be grouped according to both the overall shape and the structural configuration [432]. The basic shapes include rectangular blocks and cylinders (or disks). For the cylindrical shape, there are configurations of dense core–porous layer, less porous core–more porous layer, dense layer–porous core, and less porous layer–more porous core. For the rectangular shape, in the gradient direction, i.e., the direction with varying porosity, pore size, or composition, there are configurations of porous top–dense bottom (same as porous bottom–dense top), porous top–dense center–porous bottom, dense top–porous center–dense bottom, etc. Concerning biomedical applications, a dense core–porous layer structure is suitable for implants of a high mechanical strength and with bone ingrowth for stabilization, whereas a less porous layer–more porous core configuration can be used for drug delivery systems. Furthermore, a porous top –dense bottom structure can be shaped into implants of articulate surfaces for wear resistance and with porous ends for bone ingrowth fixation, while a dense top–porous center–dense bottom arrangement mimics the structure of head skull. Further details on bioceramics with graded porosity can be found in the literature [432].
This entry is adapted from the peer-reviewed paper https://doi.org/10.3390/coatings12101380
This entry is offline, you can click here to edit this entry!
