Chili pepper is a prominent cultivated horticultural crop that is traditionally used for food seasoning and is applied for the treatment and prevention of multiple diseases. Its beneficial health properties are due to its abundance and variety of bioactive components, such as carotenoids, capsaicinoids, and vitamins. In particular, carotenoids have important nutraceutical properties, and several studies have focused on their potential in the prevention and treatment of human diseases.
- Capsicum
- carotenoids
- chili pepper
- nutraceutical effects
- antioxidant
- cancer
- cardiovascular disorders
- anti-inflammatory
- obesity
1. Introduction
Chili pepper (Genus Capsicum, Family Solanaceae) is an important cultivated spice crop. During 2018, ≈4.2 million tons of dry chilies and peppers and ≈36.8 million tons of green chilies and peppers were produced worldwide (FAOSTATS 2018) [1]. The Capsicum genus comprises 38 different species, but only C. annuum, C. frutescens, C. chinense, C. baccatum, and C. pubescens have been domesticated [2–4].
Chili pepper fruits have abundant biochemical and mineral constituents of nutritional value. Additionally, chili peppers are good sources of bioactive compounds, such as carotenoids (lutein, β-carotene, β-cryptoxanthin, zeaxanthin, violaxanthin, capsanthin and capsorubin), vitamins C and E, and phenolic compounds, such as flavonoids (quercetin, luteolin and phenolic acids) and capsaicinoids [5–7]. The content of these bioactive compounds can vary considerably depending on the chili pepper cultivar and genotype [8].
Capsicum fruits have been used traditionally as flavoring agents and appetite stimulators, and also for the treatment of muscle pain and toothache, parasitic infections, rheumatism, wound healing, coughs and sore throat. Moreover, chili peppers also have antiseptic, antimetastatic, antifungal, antiviral, anti-inflammatory, and immunomodulatory effects, all of which are associated with their antioxidant properties [7,9].
Pungency and color are the two main characteristics of chili pepper fruits that determine their quality. Capsicum comprises pungent and non-pungent fruits with a yellow, orange, or red color (Figure 1) [2]. The diverse colors of mature pepper fruits result from the accumulation of different carotenoids in the pericarp [10]. Carotenoids are naturally occurring red, brown, orange, salmon and yellow pigments found in plants, microalgae, bacteria, archaea, and a few species of fungi and aphids [11,12].
Figure 1. Commercial bell pepper fruits of Capsicum spp. showing different colors due to the presence of (a) chlorophylls and (b–d) carotenoids.
Carotenoids are mostly 40-carbon molecules with conjugated double bonds. Based on their structures, they are classified as carotenes (containing carbon and hydrogen atoms) and xanthophylls (containing carbon, hydrogen, and oxygen), and, in general, they are lipophilic compounds and usually form hydrophobic micelles [13].
Carotenoids participate in important processes in plants such as photosynthesis, photomorphogenesis, photoprotection and development. They also serve as precursors for the biosynthesis of two kinds of plant hormones (abscisic acid and strigolactones) and a diverse set of apocarotenoids. Animals cannot synthesize carotenoids de novo, but they can get them from different foods as sources of antioxidants and provitamin A [14].
Because of their antioxidant, anti-inflammatory, and photoprotective properties, carotenoids have gained relevance, and diverse investigations have been focused on their ability to promote health. In this review, we summarize the recent advances in the nutraceutical effects and mechanism of action of carotenoids from chili pepper fruits (Table 1).
Table 1. Chili pepper fruit carotenoids and their nutraceutical effects.
|
Carotenoid |
Health Effect |
Mechanism of Action |
Reference |
|
Lutein |
Gastric cancer |
Not determined (ND) |
[15] |
|
Cancer cells |
Modulation of apoptosis and multidrug resistance |
[16] |
|
|
Edema reduction |
Reduction acetylcholinesterase, Increase seromucoids |
[17] |
|
|
Retina damage |
Modulation of oxidative stress, and pro-inflammatory gene expression |
[18] |
|
|
Macular degeneration |
Absorption of UV radiation, antioxidant |
[19] |
|
|
β-carotene |
Prostate cancer |
Inverse correlation with prostate-specific antigen (PSA) occurrence |
|
|
Gastric cancer |
ND |
[15] |
|
|
Anti-inflammatory, analgesic, antinociceptive |
ND |
[22] |
|
|
Edema reduction |
Reduction acetylcholinesterase, Increase seromucoids |
[17] |
|
|
Obesity |
Promotion of fatty acid oxidation |
[23] |
|
|
β-cryptoxanthin |
Prostate cancer |
Inverse correlation with PSA occurrence |
|
|
Gastric cancer |
ND |
[15] |
|
|
Cancer prevention |
Modulation of signaling pathways |
[24] |
|
|
Anti-inflammatory, analgesic, antinociceptive |
ND |
[22] |
|
|
Zeaxanthin |
Gastric cancer |
ND |
[15] |
|
Obesity |
Activation of AMP-activated protein (AMPK) and inhibition of lipogenesis |
[25] |
|
|
Macular degeneration |
Absorption of UV radiation, antioxidant |
[19] |
|
|
Alzheimer disease |
Inhibition of acetylcholinesterase, butyrylcholinesterase and β-secretase |
[26] |
|
|
Violaxanthin |
Cancer cells |
Modulation of apoptosis and multidrug resistance |
[16] |
|
Anti-inflammatory, analgesic, antinociceptive |
ND |
[22] |
|
|
Capsanthin |
Colon cancer |
Inhibitory effect |
[27] |
|
Skin cancer |
Chemopreventive |
[28] |
|
|
Cancer cells |
Modulation of apoptosis and multidrug resistance |
[16] |
|
|
Cancer breast (MCF-7 cells) |
Oxidative stress, DNA damage, increase p53 and Bax, lipid peroxidation |
[29] |
|
|
Atherosclerosis |
increase in the cholesterol efflux |
[30] |
|
|
Edema reduction |
Reduction of acetylcholinesterase, Increase seromucoids |
[17] |
|
|
Obesity |
Suppression of hepatic lipogenesis, fatty acid oxidation, and gluconeogenesis. Inhibit adipogenesis |
[31] |
|
|
Obesity and insulin sensitizing |
Inhibition of adipogenesis, increase of lipolytic activity, accelerated oxidation of fatty acids |
[32] |
|
|
Atherosclerosis |
Decrease on serum levels of total cholesterol, triglycerides, low density lipoprotein cholesterol, prebiotic |
[33] |
|
|
Skin health |
Counteract the cytotoxic effect of UV radiation by decreasing the formation of DNA strand breaks |
[34] |
|
|
Diabetes |
Improvement of glucose tolerance, improvement of insulin sensitivity |
[33] |
|
|
Alzheimer disease |
Inhibiting acetylcholinesterase, butyrylcholinesterase and β-secretase |
[26] |
|
|
Capsorubin |
Cancer cells |
Modulation of apoptosis and multidrug resistance |
[16] |
|
Skin health |
Counteract the cytotoxic effect of UV radiation by decreasing the formation of DNA strand breaks |
[34] |
2. Biosynthetic Pathway of Carotenoids in Chili Pepper Fruits
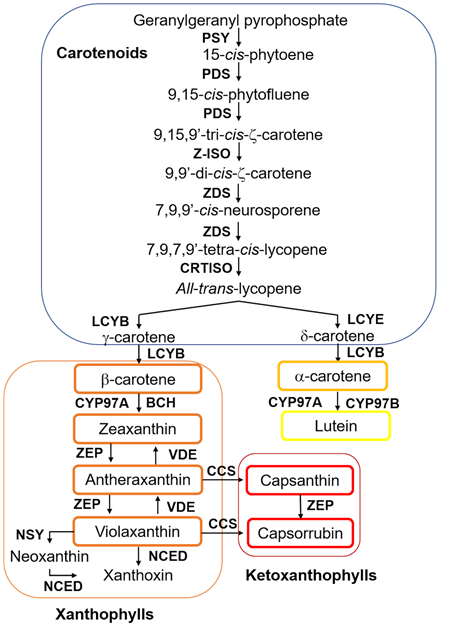
The biosynthesis of carotenoids is a conserved pathway in most plant species. Precursors of carotenoids are produced through the plastidial 2-C-methyl-d-erythritol 4-phosphate (MEP) pathway, which leads to the formation of a molecule of isopentenyl pyrophosphate (IPP), the precursor for all isoprenoids, and its isomer dimethylallyl diphosphate (DMAPP), from pyruvate and glyceraldehyde 3-phosphate. The condensation of three molecules of IPP and one molecule of DMAPP results in the formation of geranylgeranyl pyrophosphate (GGPP), a precursor not only for the biosynthesis of carotenoids but also for chlorophyll, gibberellin, phylloquinone and tocopherol production. The first step in carotenoid biosynthesis per se is the condensation of two molecules of GGPP, catalyzed by the enzyme phytoene synthase (PSY), to produce phytoene. Successively, steps of isomerization and desaturation are carried out by the phytoene desaturase (PDS), carotene desaturase (ZDS), carotene isomerase (Z-ISO), and carotenoid isomerase (CRTISO) enzymes to give rise to the formation of all-trans-lycopene (Figure 2). Then, the carotenogenic pathway separates into two branches: all-trans-lycopene can undergo cyclization by ε-lycopene cyclase (LCY-E) to produce β-carotene or can be the substrate of the β-lycopene cyclase (LCY-B) enzyme to form α-carotene. These carotenoids are then hydroxylated, and xanthophylls, such as lutein, zeaxanthin, antheraxanthin, and violaxanthin, among others, are produced (Figure 2). Xanthophyll formation is catalyzed by β-carotene hydroxylase (BCH) and cytochrome P450-type monooxygenases 97A and 97C (CYP97) [35,36].
The zeaxanthin epoxidase (ZEP) enzyme catalyzes the conversion of zeaxanthin into violaxanthin, while violaxanthin de-epoxidase (VDE) performs the reverse reaction by converting violaxanthin into zeaxanthin. Finally, violaxanthin is transformed into neoxanthin by the catalysis of neoxanthin synthase (NSY) [11,12,35,36]. The red chili pepper fruits have the capacity to accumulate two types of ketoxanthophylls, capsanthin and capsorubin, which are produced by the transformation of antheraxanthin into capsanthin and violaxanthin into capsorubin, respectively, by the action of the capsanthin-capsorubin synthase (CCS) enzyme [37].
It has been reported that the levels of the transcripts of genes encoding the two 3-hydroxy-3-methylglutaryl-CoA reductase isozymes (HMGR1 and HMGR2) that are involved in the first step of the isoprenoid pathway showed that they are not critical for carotenoid synthesis in chili pepper. In contrast, the genes encoding geranylgeranyl pyrophosphate synthase (GGPS), phytoene synthase (PSY), and phytoene desaturase (PDS) are transcribed in a sequential and coordinated manner during Capsicum fruit ripening [38]. Additionally, the evaluation of the expression profiles of genes encoding carotenogenic enzymes showed that they are expressed at 40 to 60 days after anthesis (DAA), which corresponds to the last stage of fruit maturation [39].
It has also been reported that the color of chili pepper fruits is not only related to the expression of the CCS gene, but also the specific expression profile of PSY, LCYB, CRTZ, and CCS, with different profiles resulting in the diversity of colors of Capsicum fruit. Single and multigene silencing of these genes results in different effects on the chili pepper fruit color [40].
Functional characterization and transcript quantitation of the LCYB, LCYE and CCS genes in C. annuum var. conoides showed the repression of LCYE and the induction of the LCYB and CCS genes, demonstrating an antagonistic effect on the accumulation of carotenoids in the two branches of the carotenogenic pathway in chili pepper fruit [41]. Finally, the evaluation of xanthophyll content in four Capsicum varieties at 50 DAA showed significant differences in the expression profile of the genes downstream of the carotenoid pathway [42].
Figure 2. Chili pepper fruit carotenoid biosynthetic pathway. PSY (phytoene synthase), PDS (phytoene desaturase), Z-ISO (ζ-carotene isomerase), ZDS (ζ-carotene desaturase), CRTISO (carotene isomerase), LCYB (β-lycopene cyclase), LCYE (ε-lycopene cyclase), BCH (β-carotene hydroxylase), CYP (β-carotene hydroxylase cytochrome 450 type A and B), ZEP (zeaxanthin epoxidase), VDE (violaxanthin epoxidase), CCS (capsanthin-capsorubin synthase), NSY (neoxanthin synthase), NCED (9-cis-epoxycarotenoid dioxygenase). Modified from [35,36].
An interaction was reported between geranylgeranyl diphosphate synthase (GGPPS) and the small subunit homolog protein (SSUII), a small subunit protein that shares sequence similarities with GGPPS in Capsicum annuum var. conoides. This interaction enhances GGPPS activity. Additionally, a protein-protein interaction between SSUII and PSY was described, and silencing the SSUII gene resulted in repression of the carotenogenic pathway. These findings suggest an essential role of the CaGGPPS1/CaSSUII interaction in the regulation of carotenoid biosynthesis in chili pepper fruits [43]. On the other hand, exceptionally low levels of the transcripts of the PSY and PDS genes were detected when carotenoid biosynthesis was carried out in darkness. Nevertheless, under the same conditions, only downregulation of the ZDS and LCYB genes was reported, suggesting that the conversion or degradation of carotenoids and xanthophylls is a minor process in the dark [44].
Carotenoids in plant cells are stored in chromoplasts. They are plastids that have lost photosynthetic activity due to the absence of the chlorophyll biosynthesis machinery and the presence of proteins that participate in chlorophyll degradation [45]. Chromoplasts are specialized storage organelles capable of accumulating high levels of lipids, carbohydrates, and colorful pigments in plant tissues and organs, mainly in flowers and fruits [46]. Inside the chromoplast, carotenoids are sequestered in the membrane, fibril, crystal, tubule structures and mostly in plastoglobules [47], where the formation and organization of carotenoids occur [45]. Most enzymes of the carotenoid biosynthesis pathway, with the exception of Z-ISO, have been identified in the proteomes of chili pepper chromoplasts [48]. The carotenogenic enzymes PSY2, CRTISO and NCED are located both in the stroma and the membrane of the chromoplasts. The ZDS, LCYB, and BCH enzymes are in the plastoglobules and the chromoplast membranes. PDS and ZEP have only been found in the chromoplast membranes [48–50]. Finally, significant levels of CCS gene expression were detected in the chloroplast membranes of C. annumm L. fruits, and the CCS enzyme was purified and characterized [51].
3. Carotenoid Types and Contents in Chili Pepper Fruits
Capsicum spp. fruits are abundant reservoirs of carotenoids such as lutein, β-carotene, β-cryptoxanthin, zeaxanthin, violaxanthin, capsanthin and capsorubin. Carotenoid accumulation profiles change depending on the cultivar, stage of ripening and fruit color [6]. Carotenoid quantification in fruits from five cultivars of C. annumm L. revealed that the levels of chloroplastic pigments (lutein and neoxanthin) decreased during fruit ripening and eventually almost disappeared. Additionally, the levels of antheraxanthin, β-carotene and violaxanthin increased while pigments such as β-criptoxhanthin, zeaxanthin, capsanthin-5,6-epoxide, and cucurbixanthin A were produced de novo [52]. Moreover, a study of Peruvian chili peppers showed that β-carotene was the most abundant carotenoid in almost all samples, while capsanthin was identified only in cultivars with red fruits, and only a low content of carotenoids was detected in yellow fruits [10]. An analysis of allelic variations conducted in Capsicum spp. suggested that distinct combinations of dysfunctional mutations in the PSY and CCS genes could lead to different compositions and amounts of carotenoids [53].
To elucidate the causes of the differential accumulation of carotenoids in chili pepper, several investigations have been conducted. Using fruits from contrasting Capsicum annuum variants, it was determined that there was no positive association between the accumulation of specific carotenoids and the shape of the chromoplasts. Instead, a positive correlation between the increase in β-carotene and violaxanthin contents and an increase in total carotenoid accumulation was observed. As mentioned before, the expression levels of the PSY and β-carotene hydroxylase (BCH or CrtZ-2) genes were positively correlated with the increase in the accumulation of specific carotenoids. Additionally, no association between the transcript level of CCS and the carotenoid content was observed. Finally, no positive correlation between the transcript levels of the fibrillin (which encodes an important chromoplastid structural protein involved in carotenoid retention) gene and the accumulation of capsanthin was noted. However, a positive correlation between the expression of the fibrillin gene and the violaxanthin content was observed [54]. Recent advances have demonstrated that, in C. annuum, there are different stages of the biosynthesis and accumulation of carotenoids during fruit development, and this accumulation is associated with the esterification of xanthophylls, the expression of the acyl transferase genes and the increase in the content of fibrils inside the chromoplasts [55].
The fruit color of Capsicum spp. is determined by the accumulation of specific carotenoids leading to red, brown, orange, salmon and yellow fruits [56,57]. Red chili pepper fruits accumulate the six major pigments capsanthin, β-cryptoxanthin, β-carotene, capsorubin, zeaxanthin, and antheraxanthin at variable amounts and lutein at minor amounts. A high percentage of capsanthin and capsorubin is present as fatty acid esters (monoesters and diesters) [56,58]. In red chili pepper fruits, the levels of β-carotene, β-cryptoxanthin, and zeaxanthin are very low compared with capsanthin [40]. Quantitation of 34 carotenoids analyzed by HPLC in red fruits of C. annuum var. lycopersiciforme rubrum recorded 37% capsanthin, 8% zeaxanthin, 7% cucurbitaxanthin, 3.2% capsorubin and 9% β-carotene 9%. Capsanthin 5,6-epoxide, capsanthin 3,6-epoxide, 5,6-diepikarpoxanthin, violaxanthin, antheraxanthin, cryptoxanthin, several cis isomers, and furanoid oxides were detected in lower quantities [59]. In contrast, Mini Goggal Red, a new cultivar of C. annuum L., accumulated high amounts of zeaxanthin even though its apparent color was red [60].
Thus, the intensity of the red color in chili pepper fruits is determined by the content of capsanthin and capsanthin esters. Additionally, it seems that their progenitors can play key roles in the color intensity of fruits. Moreover, subplastid fractionation demonstrated that there was a differential accumulation of pigments in lines with high and low color intensity, and PSY was the most active enzyme in the membranes of plastoglobules but was inactive in the fibril fraction [55].
The brown color of the fruits of Capsicum spp. is due to a combination of the typical red carotenoid pigments and chlorophyll B. Lutein has also been identified in brown peppers. The brown fruits of C. chinense (accession AC2212) contain a high level of chlorophyll B in combination with lutein, β-carotene, β-cryptoxanthin, zeaxanthin, antheraxanthin, capsanthin, and violaxanthin, and low levels of capsorubin [56].
On the other hand, the orange color of Capsicum fruits is due to the accumulation of red and yellow carotenoids or to the accumulation of β-carotene (orange) [61]. For instance, fruits of some varieties of chili pepper are good sources of zeaxanthin (orange) [60]. In contrast, there are orange fruits that do not accumulate any detectable levels of carotenoids located upstream in the carotenogenic pathway, such as zeaxanthin or β-carotene [56]. Orange fruits of C. chinense (accession RU 72-241) were shown to accumulate low levels of capsanthin, β-cryptoxanthin and antheraxanthin. Other Capsicum accessions with orange fruits have been found to accumulate violaxanthin and traces of β-carotene in combination with red pigments [56].
It has been reported that distinct alleles encoding carotenogenic enzymes are related to the specific profile of carotenoids in orange chili peppers. In fact, seven different alleles of the CCS gene encoding at least three variants of the enzyme have been identified in Capsicum spp [61,62]. Other cultivars of C. annuum with orange fruits have been analyzed. The chili pepper ‘Fogo’ cultivar, carrying the mutation ccs-3, is capable of producing the corresponding transcript, but no synthesis of the functional CCS enzyme was observed, and consequently, the formation of capsanthin and capsorubin was not possible. In cultivars “Orange Grande” and “Oriole”, no transcripts of CCS were detected, and no red pigments accumulated. Finally, in the Canary cultivar, transcripts of the PSY, LCYB, Crt-Z, and CCS genes were detected. Nevertheless, the CCS protein did not accumulate, and red pigments were absent [63]. An induced mutation in the gene CHY2, encoding β-carotene hydroxylase 2, resulted in the accumulation of β-carotene as the main pigment and the conversion of the fruits from red to orange [64].
In the salmon color fruits of C. chinense accession RU-72-194, only traces of esters of capsanthin, capsorubin, and α- and β-carotene were identified [56]. In the Bibas accession of C. annuum, whose fruits are yellow, there are no detectable levels of capsanthin and capsorubin. Instead, high levels of violaxanthin and its fatty acid esters and lutein were found. Chili pepper yellow fruits accumulate α-carotene, β-carotene, zeaxanthin and antheraxanthin [56,65].
A deletion of the PSY1 gene was identified in C. annuum ‘MicroPep Yellow’. In this case, the nonfunctional PSY1 gene was compensated by the PSY2 gene, leading to the yellow color of the fruits [66]. In the same way, deletion of the CCS gene is not always responsible for the yellow color of chili pepper fruits. However, different structural mutations have been identified in this gene from yellow fruits. Moreover, analysis of the promoter of the CCS gene suggests a complex transcriptional regulation of this gene in yellow chili pepper fruits [67,68].
4. Effects of Processing on the Carotenoid Content
Different methods of carotenoid extraction from plants and agro-industrial products have been performed. These methods comprise the use of solvents, enzyme-based extraction, supercritical fluid extraction, microwave-assisted extraction, Soxhlet extraction, ultrasonic extraction, and saponification. Supercritical carbon dioxide and enzyme-based extraction or the combination of two or more methods resulted in the best option to obtain high yields of carotenoids. Nevertheless, carotenoids are prone to degradation and isomerization because of the heat, light, and oxygen effects. It has been reported that capsanthin, capsorubin, and their esters are degraded at the same rate, while zeaxanthin esters respond differently to the oxidation process [69,70]. With the aim of improving the carotenoid quantity and quality, different processing techniques have been implemented and discussed.
In this sense, thermal, nonthermal, and mechanical processing have been implemented for extracting natural antioxidants from fruits and vegetables. Thermal methods such as roasting, bleaching, boiling, drying and pasteurization have been found to cause a disadvantageous effect on bioactive compounds, principally those containing pigments. However, they have been shown to protect their antioxidant effect. Nonthermal techniques such as irradiation, UV treatment, high hydrostatic pressure (HHP), and pulsed electric field (PEF) have been useful to preserve bioactive compounds, and sometimes, they have been observed to improve their antioxidant effect. In contrast, the mechanical options (chopping, trimming, peeling, crushing, slicing, sieving and pressing) have shown negative effects on the amounts, readiness and antioxidant effect of the bioactive compounds [71].
Regarding the effects of carotenoid processing, it has been reported that postharvest storage of chili pepper fruits at room temperature resulted in a gradual decrease up to 20% of the carotenoid content after one year [72]. Moreover, rapid accumulation of large amounts of phytochemicals and an attractive and intense red color was achieved when Capsicum fruits were incubated at 30 °C [73]. In concordance with that, low temperatures were found to decrease the content of free carotenoids by up to 66% after one year of storage [72]. In contrast, the combination of refrigeration and blue and UV-C stimuli was observed to promote the biosynthesis of chlorophylls and carotenoids without apparent damage to Habanero chili pepper (C. chinense) fruits during the first days of storage [74]. According to this report, red LED can accelerate the color formation and the accumulation of β-carotene, free-capsanthin, and other carotenoids in chili pepper fruits. Genes encoding the carotenogenic enzymes LCYB, CrtZ, and CCS have been reported to be upregulated by red LED, while blue LED stimulated the expression of the PSY gene. Apparently, the effect of different wavelengths of light on the accumulation of bioactive compounds is a specific characteristic of cultivars of C. annuum L. [75].
Cooking is a common process applied to fruits and vegetables that could change their content of bioactive compounds. Losses of 26% of β-carotene have been calculated during traditional preparation and cooking, and it should be noted that most of the carotenoids could be preserved during this process [76]. The effect of cooking on the concentration of bioactive compounds depends on the vegetable and fruit species and on the method of cooking. For instance, frying has been reported to decrease the content of polyphenols, flavonoids, carotenoids, and their antioxidant activity. Boiling and steaming have shown a variable impact on the stability of the same compounds depending on the fruit type. In chili pepper fruits, the frying process has been shown not to affect their carotenoid contents [77,78]. In general, it has been observed that boiling and, mostly, freezing processes decreased the carotenoid content in chili pepper fruits. Nevertheless, in some cases, high contents of β-carotene, β-cryptoxanthin and capsanthin after the same processing have been reported [79,80]. Finally, the effect of pasteurization on carotenoid content has been evaluated, and this process had a negative effect on the diversity of carotenoids contained in fruit juices [81].
The effect of nonthermal processing on bioactive compounds has also been evaluated in Capsicum spp. fruits. The drying process conserves the levels of carotenoids as well as their bioactivities [78]. Other works have concluded that milling and drying processing of chili pepper fruits decreases the content of free and esterified carotenoids up to 40% [82,83]. These contrasting results can be generated because the drying process can be influenced by factors such as the species, maturation stage, particle size of the raw material, drying temperature, and enzymatic activities, among others. In general, the traditional drying technique generates the best results with minimal carotenoid losses. Specifically, lower temperatures during drying can increase the concentration of some carotenoids in chili pepper fruits. Additionally, it has been demonstrated that the natural convective drying process can increase the content of violaxanthin in red chili pepper fruits [84,85].
The HHP process has been found to produce a positive effect on the conservation of the antioxidant activity of carotenoids, and mixing this method with thermal treatments has resulted in an increase in carotenoid bioaccessibility and antioxidant activity [86,87].
With the purpose of improving the stability of carotenoids, methods for encapsulation have been developed. Encapsulation agents such as maltodextrins, spraying, and freeze-drying are commonly used [69,88].
This entry is adapted from the peer-reviewed paper 10.3390/molecules25235573