Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
An increasing number of patients have suffered from combined heart and kidney dysfunction largely known as cardiorenal syndrome (CRS). A panel of new biomarkers (in plasma and/or urine) and artificial intelligence support systems could aid in the early identification of CRS patients at risk of developing adverse outcomes.
- cardiorenal syndrome
- biomarkers
1. Diagnosis of Acute CRS: The Need for a Panel of Multiple Biomarkers
There has been a lot of discussion concerning the controversial role of serum creatinine and eGFR in the timely diagnosis of acute kidney injury. It is clear now that serum creatinine changes become clinically obvious once severe damage has already occurred, although these changes continue to be the final gold standard indicator for renal dysfunction. In view of the known weaknesses of creatinine, researchers have turned their attention to other potential cardiorenal biomarkers.
2. Cardiac Biomarkers
The cardiac troponins, cardiac troponin T (cTnT), and cardiac troponin I (cTnI) are well-studied specific biomarkers of myocardial injury and infarction correlating with ventricular remodeling after HF and increasing with the progression of HF. Therefore, they have a role in risk stratification and prognosis in patients with HF. They also predict cardiovascular and all-cause mortality in patients with CKD [50].
The two preferred biomarkers for HF are B-type natriuretic peptide (BNP) and N-terminal probrain natriuretic peptide (NT-proBNP), released from cardiomyocytes in response to atrial stretching and evoke a natriuretic and cardioprotective role. They both correlate with HF NYHA classification, left ventricular ejection fraction (LVEF), and ventricular pressure, thus contributing to prognosis and risk stratification of patients with HF [51]. Moreover, they correlate with renal dysfunction and predict cardiovascular and all-cause mortality in CKD patients, with the NT-proBNP being more sensitive [52]. Bosselmann et al. assessed the prognostic significance of several CV biomarkers in patients with systolic dysfunction and renal dysfunction. Interestingly, it was shown that all five CV biomarkers (including cTnT, proatrial natriuretic peptide, copeptin, proadrenomedullin, and NT-proBNP) had a prognostic significance for mortality risk, that did not interact with renal dysfunction and could be interpreted independently of eGFR [53]. Copeptin (the C-terminal part of arginine vasopressin peptide) is a biomarker of cardiovascular diseases and a significant predictor of mortality in patients with myocardial infarction [54]. Adrenomedullin (ADM) is produced in the adrenal medulla, vascular endothelial cells, and in the heart in response to physical stretch and is associated with pressure and volume overload. Mid regional proadrenomedullin (MR-pro-ADM) is a more stable molecule than ADM, thus being easier to be measured. MR-pro ADM is a significant predictor of morbidity in HF and correlates with the development and progression of CKD [55].
3. Renal Biomarkers
Until now, creatinine has remained the principal biomarker of renal function that guides therapeutic decisions and determines the presence of AKI. Despite its wide use, a rise in creatinine levels follows several hours to days (depending on renal reserve or AKI extend) after the initial insult failing to timely diagnose the renal injury. Therefore, the identification of new biomarkers for the early detection of AKI has become an increasing need in clinical practice. Among identified biomarkers associated with kidney function, cystatin-C has been well studied. Cystatin-C is an endogenous cysteine proteinase inhibitor that is freely filtered in the glomerulus, completely reabsorbed by renal tubular epithelial cells and is found in urine only during tubular injury. Plasma cystatin-C can increase earlier than creatinine in early stages of AKI [56] and can detect small reductions in GFR. Apart from its role as a marker of kidney function, cystatin-C is also an independent risk factor for all-cause and cardiovascular mortality among elderly persons with or without CKD. Cystatin-C is also related to HF progression, cardiovascular events and death, thus being a potential predictor of cardiovascular complications in patients with atherosclerosis and coronary heart disease [57,58].
NGAL is a useful early marker for AKI, being able to diagnose the development of AKI up to 48 h prior to a clinical diagnosis, also correlating with AKI severity [59]. The value of serum NGAL in AHF was assessed in the AKINESIS study which found that plasma NGAL was not superior to creatinine for predicting WRF and therefore its use to diagnose AKI in AHF could not be recommended [60]. On the other hand, urinary NGAL may predict the development of WRF in AHF. Overall, the diagnostic utility of NGAL varies between different patient populations and is affected by comorbidities, timing of measurement, and cutoff values [61].
Kidney injury molecule-1 (KIM-1) is a transmembrane glycoprotein markedly expressed by the proximal tubule in response to renal injury, being a reliable predictor of AKI. It is also a predictor of disease progression in various cardiovascular diseases such as myocardial infraction and postcardiac surgery [62].
4. Other Biomarkers
C-type natriuretic peptide (CNP) together with atrial (ANP) and B-type (BNP) natriuretic peptides make up the family of natriuretic peptides, a family of hormones involved in the regulation of blood pressure, electrolyte, and volume homeostasis [63]. Both ANP and BNP have been extensively studied during the past years, whereas CNP, the ancestral precursor from which these two molecules evolved, and urinary CNP have recently attracted the attention of research as emerging biomarkers in HF and renal injury. CNP is mainly expressed in the kidney but also in cardiomyocytes, vascular endothelium, and bone [64]. Plasma levels of CNP are typically low and CNP is thought to act as an autocrine or paracrine factor. Urinary CNP is predominantly derived from local renal production and the urinary CNP excretion rate reflects renal structural integrity and function. CNP lacks significant diuretic and natriuretic effects under normal circumstances but demonstrates antiproliferative and antifibrotic properties and also exhibits a vasodilating role, thus contributing to the regulation of vascular tone [65]. Urinary CNP levels have been shown to increase in patients with AHF, suggesting an activation of the renal natriuretic peptide system in HF. An elevation of the urinary excretion of CNP is probably attributed to increased renal interstitial pressure, renal tubular injury, hypoxia, and renal fibrosis. CNP correlates with prognosis, in the setting of AHF, being able to detect renal dysfunction in HF better than urinary NGAL and KIM-1 [64]. Urinary CNP excretion may represent a marker of early renal structural remodeling and underlying renal injury both acutely and chronically [66]. CNP also has a dominant role in HF with its plasma levels been increased in this setting and correlating with a high-risk group of patients with cardiovascular comorbidities and left ventricular dysfunction.
5. Novel Diagnostic Methods
Given that CRS is a syndrome with various clinical features, correct diagnosis can be extremely challenging in such patients. Artificial intelligence using expert-driven knowledge and specialized machine-based decision trees can help significantly towards this direction. Indeed, this was shown in a paper by Dong-Ju Choi et al., where an artificial intelligence clinical decision support system (AI-CCSS) presented a high diagnostic accuracy for heart failure [67]. Further development of such AI-based tools could be of significant importance in patients with CRS, where proper and early diagnosis is the key for optimal management (Table 1 and Figure 1).
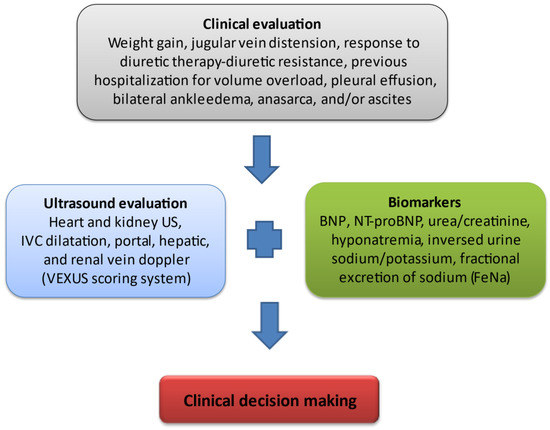
Figure 1. Assessment of venous congestion in CRS. Fluid overload has been clearly associated with adverse outcomes in critically ill patients such as end-organ damage and therefore, an increased incidence of acute kidney injury. Renal dysfunction developing in the setting of hypervolemia results from a decline in renal venous outflow and hence an increase in renal interstitial pressure. Novel methods of assessing venous congestion are much needed in order to establish effective and timely, appropriate decongestive treatment. Clinical evaluation is a key step for the assessment of volume overload including recurrent weight gain, the development of diuretic resistance (constantly increasing diuretic dose to achieve effective decongestion and/or the need for a combinatory use of diuretics with different modes of action for sequential nephron segment blockade), frequent hospitalizations in order to receive intravenous diuretic therapy, the presence of pleural effusion, peripheral oedema/anasarca, and/or ascites. Inferior vena cava (IVC) is the first venous compartment where congestion becomes apparent. Hepatic venous flow abnormality follows IVC distension with a subsequent development of portal vein pulsatility and renal venous flow Doppler abnormalities. All the above measurements constitute the VEXUS scoring system, with elevated levels if natriuretic peptides (B-type natriuretic peptide, N-terminal pro-brain natriuretic peptide) are signs of intravascular and intracardiac congestion. An increased ratio of blood urea nitrogen (BUN) to creatinine as well as dilutional hyponatremia are markers of the pathological activation of the renin–angiotensin–aldosterone system and sympathetic nervous system. The inversed urine ratio of sodium to potassium ratio as well as a constantly decreased fractional excretion of sodium in urine both depict a potential mechanism of diuretic resistance, therefore favoring the intensification of diuretic therapy.
Table 1. Important Key Points in CRS Diagnosis, Classification, and Management.
| Novel methods and biomarkers are required for accurate clinical classification of CRS. |
| Artificial intelligence support systems and clinical algorithms may be used to identify patients with CRS who are at risk of adverse outcomes. |
| Panel of novel plasma and urine biomarkers for risk stratification and for the distinction of WRF from true AKI. |
| Incorporation of improved methods of assessing venous congestion (VExUS) into routine clinical practice |
| Volume and Neurohormonal Control, SGLT2i, Inotropic support, Ultrafiltration, Iron repletion, Finerenone |
This entry is adapted from the peer-reviewed paper 10.3390/jcm12124121
This entry is offline, you can click here to edit this entry!
