Functional hypothalamic amenorrhea (FHA) is a non-organic reversible chronic endocrine disorder characterized by an impaired pulsatile secretion of the gonadotropin-releasing hormone (GnRH) from the hypothalamus. This impaired secretion, triggered by psychosocial and metabolic stressors, leads to an abnormal pituitary production of gonadotropins. As LH and FSH release is defective, the ovarian function is steadily reduced, inducing a systemic hypoestrogenic condition characterized by amenorrhea, vaginal atrophy, mood changes and increased risk of osteoporosis and cardiovascular disease. Diagnosis of FHA is made excluding other possible causes for secondary amenorrhea, and it is based upon the findings of low serum gonadotropins and estradiol (E2) with evidence of precipitating factors (excessive exercise, low weight, stress). Treatments of women with FHA include weight gain through an appropriate diet and physical activity reduction, psychological support, and integrative approach up to estrogen replacement therapy.
1. Introduction
Functional hypothalamic amenorrhea (FHA) is a reversible endocrine disorder characterized by a disturbance of the pulsatile secretion in the hypothalamus of the gonadotropin-releasing hormone (GnRH), which results in the impaired function of the hypothalamic–pituitary–ovarian axis, chronic anovulation and hypoestrogenism [
1]. It is responsible for approximately 25–35% and 3% of secondary and primary amenorrhea cases, respectively [
2].
Risk factors for FHA include low-weight eating disorders (up to anorexia nervosa) and other causes, such as a low body weight due to excessive exercise and stress [
3]. FHA often occurs in female athletes. Indeed the combination of low energy availability, hypothalamic–pituitary–gonadal axis inhibition resulting in menstrual dysfunction, and low bone density is called the “female athlete triad” [
4].
Psychosocial and metabolic stressors, including eating disorders and excessive exercise, induce various hormonal changes that impair hypothalamic secretion of GnRH, leading to an abnormal pituitary production of gonadotropins and to the failure of follicle recruitment, thus leading to anovulation and hypoestrogenism [
5].
GnRH–luteinizing hormone (LH) disturbances in FHA include a wide range of features: both a lower and higher mean frequency of LH pulses, complete absence of LH pulsatility and normal-appearing secretion pattern [
6]. The reduction in GnRH drive results in LH and FSH levels that are too low to stimulate full folliculogenesis and ovulatory ovarian function [
7].
All these changes in FHA patients may be considered a natural protective mechanism that temporarily suppresses reproductive functions when physical conditions are not suitable to sustaining a pregnancy [
8].
In FHA, as a consequence of energy deficiency from under-nutrition or from excessive energy use, a cascade of energy-conservation mechanisms take place, such as reduction of the concentrations of glucose, insulin, leptin, insulin-like growth factor (IGF-1) and kisspeptin (Kp), or the elevation of growth hormone (GH), neuropeptide Y, ghrelin, beta-endorphin and cortisol plasma levels [
9].
The occurrence of a hypoestrogenic condition in FHA negatively affects most of the estrogen-sensitive organs. It is best to resolve this within a reasonable span of time (a few months), as otherwise fertility issues as well as an increased risk of osteoporosis and cardiovascular disease might occur. In addition, women with FHA may experience symptoms such as vaginal atrophy and mood changes [
10].
2. Diagnosis of FHA
Functional hypothalamic amenorrhea should be differentiated from other forms of primary or secondary amenorrhea. The diagnosis of FHA typically involves a combination of a medical history, physical examination and laboratory tests. The diagnostic process should also consider other potential causes of amenorrhea, such as pregnancy, thyroid dysfunction, or polycystic ovary syndrome (PCOS) [
11]. Diagnosis of FHA is based upon the findings of some clinical and laboratory issues, such as amenorrhea, low serum gonadotropins and estradiol (E2), with evidence of a precipitating factor (exercise, low weight, stress).
Before the onset of amenorrhea, women with FHA have normal cycles, which become irregular and then cease after loss of weight, increased exercise activity or significant stress occur together or one after another. In some women, menses may stop without a preceding period of oligomenorrhea. If a patient had exercise-induced amenorrhea in the past that remitted when she decreased exercising, it is likely to recur if she resumes exercising without a compensatory caloric increase [
7].
When a hypogonadotropic hypogonadism is evidenced, the key diagnostic tool is a GnRH stimulation test, which in the case of FHA shows a positive response of the gonadotropins to exogenous GnRH [
12]. Such a test is relevant for a correct diagnosis in those cases where amenorrhea occurred just after menarche, thus permitting one to differentiate a delayed puberty from a FHA.
The administration of a progestin such as dihydrogesterone for 10 days, usually named as “progestin challenge test”, may be useful, since FHA is associated with scant or no withdrawal bleeding as E2 levels are deficient. In conditions such as PCOS, in which E2 levels are only relatively low, bleeding usually occurs following the administration of exogenous progestin. An imaging study to assess the internal genitalia may be of value in adolescents with primary amenorrhea before the progestin challenge test [
7].
Once the hypothalamic origin has been found, it is important to rule out genetic diseases, such as Kallman syndrome (characterized by anosmia) or Prader–Willi syndrome (with characteristic hyperphagia, obesity and retardation). Features such as delayed puberty, primary amenorrhea and the presence of additional symptoms (anosmia, mental retardation, extreme obesity, facial dysmorphia, malabsorption) are suggestive of congenital diseases [
13]. Imaging evaluation should be performed to exclude organic diseases of the hypothalamic area (neoplasms, tuberculosis, parasitosis, sarcoidosis) [
11].
If amenorrhea is present for more than six months, or earlier in case of a suspicion of severe nutritional deficiency, other energy deficit states or a history of fragility fractures, a baseline bone mineral density (BMD) should be performed [
14].
3. Treatment of Functional Hypothalamic Amenorrhea
Treatment of women with FHA includes treatment of the underlying cause of hypogonadotropic hypogonadism (energy deficit from insufficient caloric intake, excessive exercise or emotional stress) and its consequences, such as low BMD, anovulatory oligo-amenorrhea, and infertility or genitourinary symptoms (vaginal dryness and dyspareunia) due to estrogen deficiency [
7].
Patient treatment for women with FHA should consider whether severe bradycardia, hypotension, orthostasis, and/or electrolyte imbalance is present [
15].
Low energy availability leads to hypothalamic–pituitary–ovarian (HPO) axis disruption as a defensive mechanism to save energy, as reflected in menstrual dysfunction. Energy availability can be described as the difference between energy intake and exercise energy expenditure, normalized to fat-free mass [
4]. Weight gain through refeeding and improved energy availability in amenorrhoeic patients with anorexia nervosa correlated with the resumption of menses [
16]. The approach should include dietary evaluation and counselling as well as psychological support for treating stress and enhancing behavioral change [
17].
It is important to note that amenorrhea may persist for some time after the reversal of precipitating factors and that at least up to 6 to 12 months of weight stabilization may be required for the resumption of menses. Golden et al. suggested that a weight gain of 2.0 kg more than the weight at which menses stopped was needed for the restoration of menses [
18].
In some cases, even after weight stabilization, regular menses never resume. This suggests that factors other than nutrition play a key role in FHA pathophysiology, such as stress or other psychological disorders. In comparison to eumenorrheic women, women with FHA have been observed to exhibit a higher incidence of dysfunctional attitudes, struggle to cope with daily stresses and have a history of mental health issues and mood disorders [
19]. In these patients, psychological support, such as cognitive behavioral therapy (CBT), shows the capacity to restore ovarian function and also alter metabolic function, improving cortisol, leptin, and TSH [
20].
Estrogen replacement therapy may be considered after 6 to 12 months of nutritional, psychological, and exercise-related interventions in those with a low bone density and/or evidence of skeletal fragility.
The Endocrine Society’s 2017 Clinical Practice Guideline for the diagnosis and treatment of FHA advises against the use of oral contraceptive pills (OCPs) for the sole purpose of regaining menses or improving BMD, since OCPs may hide bone loss not being so effective on bone mass deposition [
7].
The use of transdermal E2 therapy with cyclic oral progestin should be preferred over oral hormonal therapies, as it has been demonstrated to improve lumbar and hip BMD in patients with FHA [
21]. Transdermal estrogen likely has a more positive effect on BMD than OCPs because it does not affect IGF-I secretion, a bone-trophic hormone that is downregulated by OCPs [
22,
23].
The use of bisphosphonates, denosumab, testosterone, and leptin to improve BMD in adolescents and women with FHA is not recommended [
7].
In patients with FHA wishing to conceive, after a complete fertility work-up, induction of ovulation should be attained. As a first-line treatment, pulsatile GnRH followed by gonadotropin therapy should be chosen [
7].
In most patients with FHA, exogenous GnRH or exogenous gonadotropin would likely be efficacious for inducing ovulation, as pituitary–ovarian feedback mechanisms are intact. This technique leads to more physiologic ovulatory menstrual cycles with monofollicular development and minimal risk of developing multiple pregnancies [
24,
25], (
Figure 1).
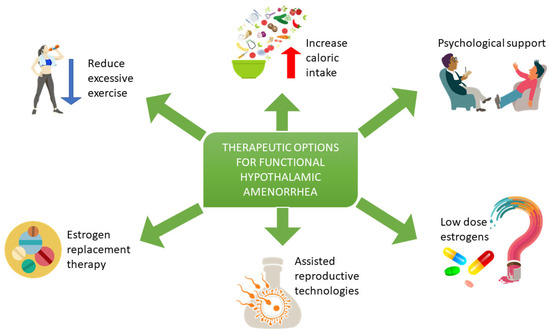
Figure 1. Treatment of women with FHA includes a reduction of excessive exercise, dietary evaluation and psychological support to reduce stress, an enhancement of behavioral change and an increase of energy availability. Estrogen replacement therapy may be considered after 6 to 12 months of nutritional, psychological, and exercise-related interventions in those with low bone density and/or evidence of skeletal fragility. Assisted reproductive technologies may be considered in patients wishing to conceive. Low-dose estrogen use in patients with FHA is still under study.
Up to now, there have been no randomized clinical trials that have evaluated the use of clomiphene citrate, an estrogen receptor antagonist, in inducing ovulation and treating infertility in women with FHA. In fact, these patients are characterized by low estrogen levels, and they do not show the activation of the estrogen-induced negative feedback. In addition, Djurovic et al. reported that after 10 days of treatment with clomiphene citrate, menstrual bleeding occurred in only 9 out of 17 patients who recovered a normal body weight but not in those who showed a diagnosis of anorexia nervosa [
26]. Therefore, treatment with clomiphene citrate may be considered if a woman has sufficient endogenous estrogen levels [
27].
A BMI of at least 18.5 kg/m
2 is considered to be the minimal threshold that a woman needs to optimize her chances for fertility. Moreover, an extremely low BMI is associated with a higher risk of adverse pregnancy outcomes [
28]. Therefore, induction of ovulation should be limited to women with a satisfactory body weight.
AMH plasma levels can be used to estimate ovarian reserve in women with hypothalamic hypogonadism [
29], as gonadotropins will be low and the identification of primary ovarian insufficiency (POI) may be delayed due to the fact that FHA induces a reduced gonadotropin output [
29].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11061763