Nanocelluloses (NCs) are appealing nanomaterials that have experienced rapid development, with great potential in the biomedical field. This trend aligns with the increasing demand for sustainable materials, which will contribute both to an improvement in wellbeing and an extension of human life, and with the demand to keep up with advances in medical technology.
- nanocellulose
- biological properties
- tissue engineering
- drug delivery
- wound dressing
1. Introduction
|
Type |
Sources |
Preparation Methods |
Special Characteristics |
References |
|---|---|---|---|---|
|
CNCs |
Wood, cotton, hemp, wheat straw, tunicin, algae, bacteria |
Acid hydrolysis |
Short, rigid nanocrystals; woods (W/L): 5–40 nm/100–300 nm; non-woods (W/L): 7–25 nm/84–800 nm (cotton, wheat); 12–21 nm/107–215 nm (ramie); 25–30 nm/300–400 nm (BNC) |
|
|
CNFs |
Wood, sugar, beet, potato tuber, hemp, flax |
Delamination before/after chemical or enzymatic treatment |
Long, flexible nanofibers with significant amorphous content; web-like structures; W/L: 20–100 nm/several µm. |
|
|
BNC |
Low-molecular-weight sugars and alcohols |
Bacterial synthesis |
Highly crystalline 3D network, high purity; aggregated into nanofibrillar bundles; W = 50–150 nm; Static fermentation: uniaxially oriented ribbons; agitated fermentation: disordered, overlapping ribbon-like morphology. |
2. Sources for Nanocellulose Materials
3. Isolation Methods
3.1. CNCs
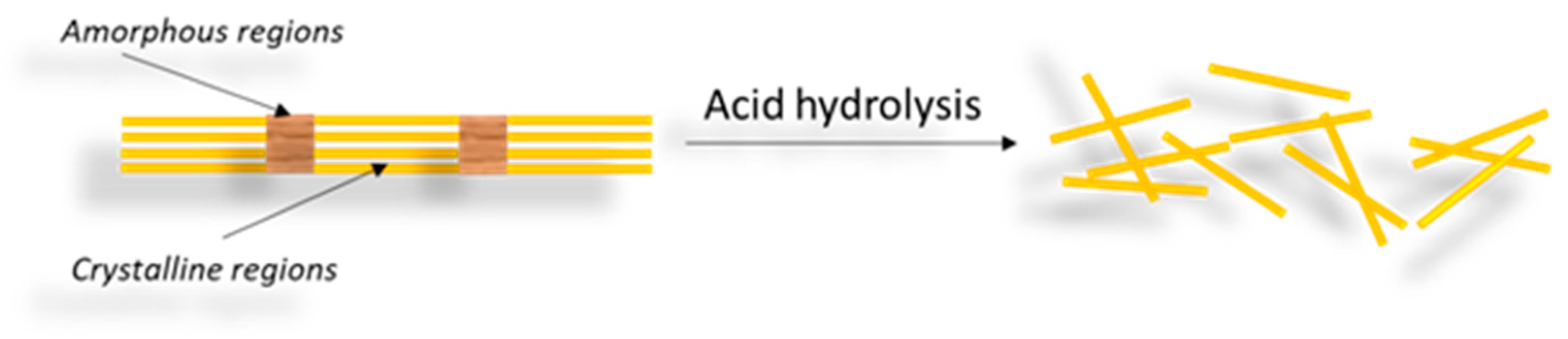
|
Source |
Preparation Technique |
Preparation Conditions |
References |
|---|---|---|---|
|
Bleached hardwood pulp |
Acid hydrolysis |
75% PTA/90 °C/30 h |
[12] |
|
Black spruce |
Periodate oxidation |
NaIO4/room T/105 rpm/96 h |
[13] |
|
Whatman ashless filter paper |
Acid hydrolysis |
85% H3PO4/50 min/100 °C |
[44] |
|
Acid hydrolysis |
64 wt% H2SO4/45 °C/45 min |
[14] |
|
|
Acid hydrolysis TEMPO-oxidation |
2.5 M HCl/70 °C/2 h TEMPO/NaClO |
[15] |
|
|
Tunicates |
Enzymatic hydrolysis |
Novozym 476 (20 FPU/g); 50 °C/2 h |
[31] |
|
TEMPO-oxidation |
TEMPO/NaBr/NaClO |
||
|
Acid hydrolysis |
55 wt% H2SO4/60 °C/20 min |
||
|
Red algae |
Acid hydrolysis |
64 wt% H2SO4/45 °C/45 min |
[36] |
|
Barley straw |
Acid hydrolysis |
64% H2SO4/50 °C/75 min |
[16] |
|
Ramie fibers |
Acid hydrolysis |
16 M H3PO4/150 °C/90 min |
[48] |
|
41–50% H2SO4/45 °C/30 min |
[17] |
||
|
Bacterial cellulose |
Acid hydrolysis |
50% H2SO4/50 °C/40 min |
[18] |
Abbreviations: PTA—Phosphotungstic acid; NaIO4—Sodium (meta) periodate; H3PO4—Phosphoric acid; H2SO4—Sulfuric acid; TEMPO—(2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl.
3.2. CNFs
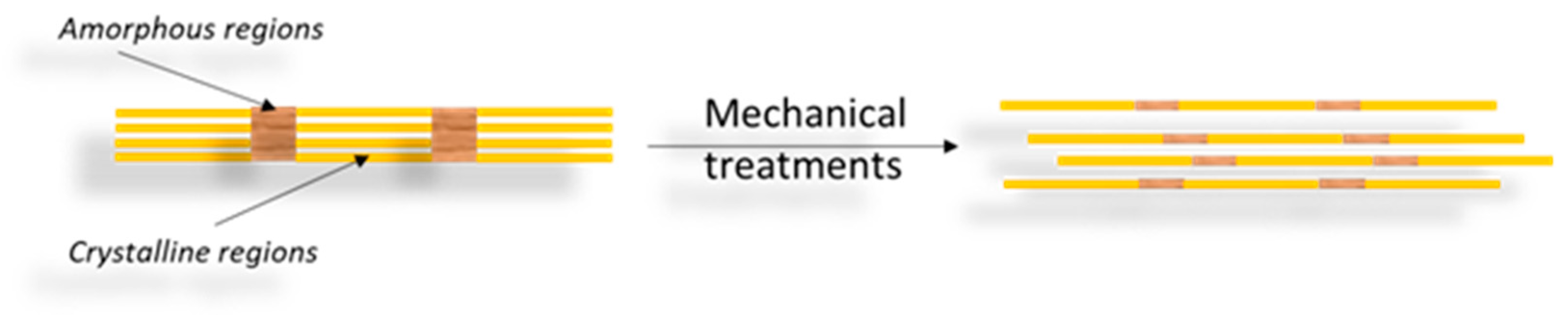
|
Source |
Preparation Technique |
Preparation Conditions |
References |
|---|---|---|---|
|
Bagasse |
a. Enzyme pretreatment b. Mechanical grinding |
a. Novozymes endoglucanase/50 °C/12 h b. Ultrafine grinder, 10–15 J/1500 rpm |
[55] |
|
Cassava roots |
a. Alkaline treatment b. Acid hydrolysis |
a. 5% KOH/25 °C/14 h b. 30% SA/90 min/60 °C |
[66] |
|
a. Alkaline treatment b. TEMPO-oxidation |
a. 5% KOH/25 °C/14 h b. TEMPO/NaBr/NaClO |
||
|
Waste hemp |
a. Alkali/bleaching treatment b. Acid hydrolysis |
a. 2 wt/V% NaOH/50 °C/3 h; NaClO b. 64% wt/wt SA/45 °C/30 min |
[67] |
|
a. Alkali/bleaching treatment b. Acid hydrolysis c. Ultrasonication |
a. 4 wt% NaOH/80 °C/2 h; 1.7 wt% NaClO2/ABS (pH 4.8)/1 h/100 °C; b. 45%, 64% SA, FA, MA/60, 90 min/45°, 65 °C; c. 4 min/low speed. |
[56] |
|
|
Kenaf |
a. Formic acid/acetic acid; b. Peroxyformic acid/ peroxyacetic acid c. Bleaching treatment d. Ball milling |
a. 85% FA/AA/110 °C/2 h; b. 35% H2O2 with 85% FA/AA/80 °C/2 h; c. 35% H2O2/NaOH/80 °C/2 h; d. 30, 60, 90, 120 min. |
[70] |
|
Wheat straw |
|||
|
Carrots residue |
a. Blanching b. Refining c. Homogenization |
a. 80 °C/1 h; b. PFI mill to 10,000 revolutions; c. Homogenizer: 2 wt%/5 passes/1000 bar. |
[71] |
|
Sugar beet |
a. Steam Explosion b. Bleaching c. Ultrasonication |
a. 220 °C/35 min/2.4 MPa; b. 6 wt% H2O2/80 °C/24 h; c. Ice/water bath/30 min/1000 W. |
[72] |
Abbreviations: SA—Sulfuric acid; FA—Formic acid; AA—acetic acid; MA—Maleic acid; PFA—Peroxyformic acid; PAA—peroxyacetic acid; TEMPO—2,2,6,6-tetramethylpiperidin-1-oxyl; ABS—acetate buffer solution.
3.3. BNC
|
Bacteria Strain |
Fermentation Technique |
Carbon Source |
Optimal Fermentation Conditions |
Productivity, g/L/day |
Ref. |
|---|---|---|---|---|---|
|
G. xylinus ATCC 700178 |
Static |
Carob/Haricot bean |
2.5 g/L carbon, T = 30 °C, pH = 5.5, t = 9 days |
0.19 |
[82] |
|
G. xylinus KCCM 41431 |
Static |
Residual crude glycerol |
20 g/L glycerol, pH = 5, t = 7 days |
0.99 |
[86] |
|
G. xylinus PTCC 1734 |
Static |
Beet molasses/Cheese whey |
T = 28 °C, pH = 5.5, t = 14 days |
0.32 |
[18] |
|
G. sucrofermentans B-11267 |
Dynamic |
Wheat vinasse /Cheese whey |
T = 28 °C, pH = 3.95–4.96, t = 3 days, 250 rpm |
2.06 |
[85] |
|
A. xylinum ATCC 23767 |
Static |
Waste extract tobacco |
T = 30 °C, pH = 6.5, t = 7 days |
0.32 |
[87] |
|
Dynamic |
T = 30 °C, 150 rpm |
0.74 |
|||
|
K. europaeus SGP37 |
Static batch/Static intermittent fed-batch |
Sweet lime pulp waste |
T = 30 °C, pH = 6, t = 16 days; Addition every 48 h and 96 h |
0.40 |
[88] |
|
K. xylinus PTCC 1734 |
Static |
Vinasse |
40% vinasse, T = 30 °C, pH = 6, t = 10 days |
0.18 |
[89] |
|
K. xylinus PTCC 1734 |
Dynamic |
Date syrup/Cheese whey |
Date syrup: cheese whey ratio = 50:50, T = 28 °C, pH = 4.48, t = 10 days |
0.19 |
[84] |
Abbreviations: G. xylinus—Gluconacetobacter xylinus; A. xylinum—Acetobacter xylinum; K. xylinus—Komagatacibacter xylinus; K. europaeus—Komagataeibacter europaeus.
This entry is adapted from the peer-reviewed paper 10.3390/ma16124447
References
- Barhoum, A.; Rastogi, V.K.; Mahur, B.K.; Rastogi, A.; Abdel-Haleem, F.M.; Samyn, P. Nanocelluloses as new generation materials: Natural resources, structure-related properties, engineering nanostructures, and technical challenges. Mater. Today Chem. 2022, 26, 101247.
- Nehra, P.; Chauhan, R.P. Eco-friendly nanocellulose and its biomedical applications: Current status and future prospect. J. Biomater. Sci. Polym. Ed. 2021, 32, 112–149.
- Ahankari, S.S.; Subhedar, A.R.; Bhadauria, S.S.; Dufresne, A. Nanocellulose in food packaging: A review. Carbohydr. Polym. 2021, 255, 117479.
- Shak, K.P.Y.; Pang, Y.L.; Mah, S.K. Nanocellulose: Recent advances and its prospects in environmental remediation. Beilstein J. Nanotechnol. 2018, 9, 2479–2498.
- Sabo, R.; Yermakov, A.; Law, C.T.; Elhajjar, R. Nanocellulose-enabled electronics, energy harvesting devices, smart materials and sensors: A review. J. Renew. Mater. 2016, 4, 297–312.
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41719–41737.
- Maiuolo, L.; Algieri, V.; Olivito, F.; Tallarida, M.A.; Costanzo, P.; Jiritano, A.; De Nino, A. Chronicle of nanocelluloses (NCs) for catalytic applications: Key advances. Catalysts 2021, 11, 96.
- Mirtaghavi, A.; Luo, J.; Muthuraj, R. Recent advances in porous 3D cellulose aerogels for tissue engineering applications: A review. J. Compos. Sci. 2020, 4, 152.
- Mokhena, T.C.; John, M.J. Cellulose nanomaterials: New generation materials for solving global issues. Cellulose 2020, 27, 1149–1194.
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for ground breaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748.
- Ioelovich, M. Characterization of various kinds of nanocellulose. In Handbook of Nanocellulose and Cellulose Nanocomposites, 1st ed.; Kargarzadeh, H., Ahmad, I., Thomas, S., Dufresne, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; Chapter 2; pp. 51–100.
- Liu, Y.; Wang, H.; Yu, G.; Yu, Q.; Li, B.; Mu, X. A novel approach for the preparation of nanocrystalline cellulose by using phosphotungstic acid. Carbohydr. Polym. 2014, 110C, 415–422.
- Yang, H.; Chen, D.; van de Ven, T.G.M. Preparation and characterization of sterically stabilized nanocrystalline cellulose obtained by periodate oxidation of cellulose fibers. Cellulose 2015, 22, 1743–1752.
- Hu, Z.; Cranston, E.D.; Ng, R.; Pelton, R. Tuning cellulose nanocrystal gelation with polysaccharides and surfactants. Langmuir 2014, 30, 2684–2692.
- Pereira, P.H.F.; Waldron, K.W.; Wilson, D.R.; Cunha, A.P.; de Brito, E.S.; Rodrigues, T.H.S.; Rosa, M.F.; Azeredo, H.M.C. Wheat straw hemicelluloses added with cellulose nanocrystals and citric acid. Effect on film physical properties. Carbohydr. Polym. 2017, 164, 317–324.
- Oun, A.A.; Rhim, J.W. Isolation of cellulose nanocrystals from grain straws and their use for the preparation of carboxymethyl cellulose-based nanocomposite films. Carbohydr. Polym. 2016, 150, 187–200.
- Listyanda, R.F.; Kusmono; Wildan, M.W.; Ilman, M.N. Extraction and characterization of nanocrystalline cellulose (NCC) from ramie fiber by sulphuric acid hydrolysis. AIP Conf. Proc. 2020, 2217, 030069.
- Salari, M.; Khiabani, M.S.; Mokarram, R.R.; Ghanbarzadeh, B.; Kafil, H.S. Preparation and characterization of cellulose nanocrystals from bacterial cellulose produced in sugar beet molasses and cheese whey media. Int. J. Biol. Macromol. 2019, 122, 280–288.
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiyah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625.
- Trache, D. Nanocellulose as a promising sustainable material for biomedical applications. AIMS Mater. Sci. 2018, 5, 201–205.
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From fundamentals to advanced applications. Front. Chem. 2020, 8, 392–424.
- Xue, Y.; Mou, Z.; Xiao, H. Nanocellulose as sustainable biomass material: Structure, properties, present status and future prospects in biomedical applications. Nanoscale 2017, 9, 14758–14781.
- Sharma, C.; Bhardwaj, N.K. Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Mater. Sci. Eng. C 2019, 104, 109963.
- Sanyang, M.L.; Saba, N.; Jawaid, M.; Mohammad, F.; Salit, M.S. Bacterial nanocellulose applications for tissue engineering. In Nanocellulose and Nanohydrogel Matrices: Biotechnological and Biomedical Applications, 1st ed.; Jawaid, M., Mohammad, F., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2017; Chapter 3; pp. 47–66.
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466.
- Khalil, H.P.S.A.; Adnan, A.S.; Yahya, E.B.; Olaiya, N.G.; Safrida, S.; Hossain, M.S.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.K.; Oyekanmi, A.A.; et al. A review on plant cellulose nanofibre-based aerogels for biomedical applications. Polymers 2020, 12, 1759.
- Pachuau, L. Application of nanocellulose for controlled drug delivery. In Nanocellulose and Nanohydrogel Matrices: Biotechnological and Biomedical Applications, 1st ed.; Jawaid, M., Mohammad, F., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2017; Chapter 1; pp. 1–19.
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969.
- Raghav, N.; Sharma, M.R.; Kennedy, J.F. Nanocellulose: A mini-review on types and use in drug delivery systems. Carbohydr. Polym. Technol. Appl. 2021, 2, 100031.
- Hamad, W.Y. Cellulose nanocrystals and nanofibrils in advanced applications. In Handbook of Nanocellulose and Cellulose Nanocomposites, 1st ed.; Kargarzadeh, H., Ahmad, I., Thomas, S., Dufresne, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2017; Chapter 24; pp. 799–832.
- Zhao, Y.; Zhang, Y.; Lindström, M.E.; Li, J. Tunicate cellulose nanocrystals: Preparation, neat films and nanocomposite films with glucomannans. Carbohydr. Polym. 2015, 117, 286–296.
- Samiee, S.; Ahmadzadeh, H.; Hosseini, M.; Lyon, S. Algae as a source of microcrystalline cellulose. In Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts; Hosseini, M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; Chapter 17; pp. 331–350.
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786.
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M. Microcrystalline cellulose from Posidonia oceanica brown algae: Extraction and characterization. Int. J. Biol. Macromol. 2019, 138, 837–845.
- El Achaby, M.; El Miri, N.; Hannache, H.; Gmouh, S.; Ben youcef, H.; Aboulkas, A. Production of cellulose nanocrystals from vine shoots and their use for the development of nanocomposite materials. Int. J. Biol. Macromol. 2018, 117, 592–600.
- Chen, Y.W.; Leea, H.V.; Juana, J.C.; Phang, S.M. Production of new cellulose nanomaterial from red algae marine biomass Gelidium elegans. Carbohydr. Polym. 2016, 151, 1210–1219.
- Bettaieb, F.; Khiari, R.; Dufresne, A.; Mhenni, M.F.; Putaux, J.L.; Boufi, S. Nanofibrillar cellulose from Posidonia oceanica: Properties and morphological features. Ind. Crops Prod. 2015, 72, 97–106.
- Ludwicka, K.; Kaczmarek, M.; Białkowska, A. Bacterial nanocellulose—A biobased polymer for active and intelligent food packaging applications: Recent advances and developments. Polymers 2020, 12, 2209.
- Ferreira, F.V.; Pinheiro, I.F.; de Souza, S.F.; Mei, L.H.I.; Lona, L.M.F. Polymer composites reinforced with natural fibers and nanocellulose in the automotive industry: A short review. J. Compos. Sci. 2019, 3, 51.
- Halib, N.; Perrone, F.; Cemazar, M.; Dapas, B.; Farra, R.; Abrami, M.; Chiarappa, G.; Forte, G.; Zanconati, F.; Pozzato, G.; et al. Potential applications of nanocellulose-containing materials in the biomedical field. Materials 2017, 10, 977.
- Tan, T.H.; Lee, H.V.; Dabdawb, W.A.Y.; Abd Hamid, S.B.B.O. A review of nanocellulose in the drug-delivery system. In Materials for Biomedical Engineering: Nanomaterials-Based Drug delivery, 1st ed.; Holban, A.M., Grumezescu, A.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Chapter 5; pp. 131–164.
- Kontturi, E.; Laaksonen, P.; Linder, M.B.; Nonappa; Gröschel, A.H.; Rojas, O.J.; Ikkala, O. Advanced materials through assembly of nanocelluloses. Adv. Mater. 2018, 30, 1703779.
- Liu, C.; Li, B.; Du, H.; Lv, D.; Zhang, Y.; Yu, G.; Mu, X.; Peng, H. Properties of nanocellulose isolated from corncob residue using sulfuric acid, formic acid, oxidative and mechanical methods. Carbohydr. Polym. 2016, 151, 716–724.
- Camarero-Espinosa, S.; Kuhnt, T.; Foster, E.J.; Weder, C. Isolation of thermally stable cellulose nanocrystals by phosphoric acid hydrolysis. Biomacromolecules 2013, 14, 1223–1230.
- Peyre, J.; Pääkkönen, T.; Reza, M.; Kontturi, E. Simultaneous preparation of cellulose nanocrystals and micron-sized porous colloidal particles of cellulose by TEMPO-mediated oxidation. Green Chem. 2015, 17, 808–811.
- Sirviö, J.A.; Visanko, M.; Laitinen, O.; Ämmälä, A.; Liimatainen, H. Amino-modified cellulose nanocrystals with adjustable hydrophobicity from combined regioselective oxidation and reductive amination. Carbohydr. Polym. 2016, 136, 581–587.
- Satyamurthy, P.; Jain, P.; Balasubramanya, R.H.; Vigneshwaran, N. Preparation and characterization of cellulose nanowhiskers from cotton fibres by controlled microbial hydrolysis. Carbohydr. Polym. 2011, 83, 122–129.
- Kusmono, K.; Affan, M.N. Isolation and characterization of nanocrystalline cellulose from Ramie fibers via phosphoric acid hydrolysis. J. Nat. Fibers 2020, 19, 2744–2755.
- Murizan, N.I.S.; Mustafa, N.S.; Ngadiman, N.H.A.; Yusof, N.M.; Idris, A. Review on nanocrystalline cellulose in bone tissue engineering applications. Polymers 2020, 12, 2818.
- Levanič, J.; Petrovič Šenk, V.; Nadrah, P.; Poljanšek, I.; Oven, P.; Haapala, A. Analyzing TEMPO-oxidized cellulose fiber morphology: New insights into optimization of the oxidation process and nanocellulose dispersion quality. ACS Sustain. Chem. Eng. 2020, 8, 17752–17762.
- Isogai, A.; Hänninen, T.; Fujisawa, S.; Saito, T. Review: Catalytic oxidation of cellulose with nitroxyl radicals under aqueous conditions. Prog. Polym. Sci. 2018, 86, 122–148.
- Sofla, M.R.K.; Batchelor, W.; Kosinkova, J.; Pepper, R.; Brown, R.; Rainey, T. Cellulose nanofibres from bagasse using a high speed blender and acetylation as a pretreatment. Cellulose 2019, 26, 4799–4814.
- Onyianta, A.J.; Dorris, M.; Williams, R.L. Aqueous morpholine pre-treatment in cellulose nanofibril (CNF) production: Comparison with carboxymethylation and TEMPO oxidisation pre-treatment methods. Cellulose 2018, 25, 1047–1064.
- Malucelli, L.C.; Matos, M.; Jordão, C.; Lomonaco, D.; Lacerda, L.G.; Carvalho Filho, M.A.S.; Magalhães, W.L.E. Influence of cellulose chemical pretreatment on energy consumption and viscosity of produced cellulose nanofibers (CNF) and mechanical properties of nanopaper. Cellulose 2019, 26, 1667–1681.
- Liu, X.; Jiang, Y.; Qin, C.; Yang, S.; Song, X.; Wang, S.; Li, K. Enzyme-assisted mechanical grinding for cellulose nanofibers from bagasse: Energy consumption and nanofiber characteristics. Cellulose 2018, 25, 7065–7078.
- Mhlongo, J.T.; Nuapia, Y.; Motsa, M.M.; Mahlangu, T.O.; Etale, A. Green chemistry approaches for extraction of cellulose nanofibers (CNFs): A comparison of mineral and organic acids. Mater. Today Proc. 2022, 62, S57–S62.
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses, and commercial potential. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 815–827.
- Zdunek, A.; Kozioł, A.; Pieczywek, P.M.; Cybulska, J. Evaluation of the nanostructure of pectin, hemicellulose and cellulose in the cell walls of pears of different texture and firmness. Food Bioprocess Technol. 2014, 7, 3525–3535.
- Chirayil, C.J.; Joy, J.; Mathew, L.; Mozetic, M.; Koetz, J.; Thomas, S. Isolation and characterization of cellulose nanofibrils from Helicteresisora plant. Ind. Crops Prod. 2014, 59, 27–34.
- Okahisa, Y.; Furukawa, Y.; Ishimoto, K.; Narita, C.; Intharapichai, K.; Ohara, H. Comparison of cellulose nanofiber properties produced from different parts of the oil palm tree. Carbohydr. Polym. 2018, 198, 313–319.
- Harini, K.; Ramya, K.; Sukumar, M. Extraction of nano cellulose fibers from the banana peel and bract for production of acetyl and lauroyl cellulose. Carbohydr. Polym. 2018, 201, 329–339.
- Tibolla, H.; Pelissari, F.M.; Rodrigues, M.I.; Menegalli, F.C. Cellulose nanofibers produced from banana peel by enzymatic treatment: Study of process conditions. Ind. Crops Prod. 2017, 95, 664–674.
- Khawas, P.; Deka, S.C. Isolation and characterization of cellulose nanofibers from culinary banana peel using high-intensity ultrasonication combined with chemical treatment. Carbohydr. Polym. 2016, 137, 608–616.
- Pereira, A.L.S.; do Nascimento, D.M.; Filho, M.M.S.; Morais, J.P.S.; Vasconcelos, N.F.; Feitosa, J.P.A.; Brígida, A.I.S.; Rosa, M.F. Improvement of polyvinyl alcohol properties by adding nanocrystalline cellulose isolated from banana pseudostems. Carbohydr. Polym. 2014, 112, 165–172.
- Kouadri, I.; Satha, H. Extraction and characterization of cellulose and cellulose nanofibers from Citrullus colocynthis seeds. Ind. Crops Prod. 2018, 124, 787–796.
- Czaikoski, A.; Lopes da Cunha, R.; Menegalli, F.C. Rheological behavior of cellulose nanofibers from cassava peel obtained by combination of chemical and physical processes. Carbohydr. Polym. 2020, 248, 116744.
- Padinjakkara, A.; Scarinzi, G.; Santagata, G.; Malinconico, M.; Razal, J.M.; Thomas, S.; Salim, N.V. Enhancement of adhesive strength of epoxy/carboxyl-terminated poly(butadiene-co-acrylonitrile) nanocomposites using waste hemp fiber-derived cellulose nanofibers. Ind. Eng. Chem. Res. 2020, 59, 10904–10913.
- Pacaphol, K.; Aht-Ong, D. Preparation of hemp nanofibers from agricultural waste by mechanical defibrillation in water. J. Clean. Prod. 2017, 142, 1283–1295.
- Leite, A.L.M.P.; Zanon, C.D.; Menegalli, F.C. Isolation and characterization of cellulose nanofibers from cassava root bagasse and peelings. Carbohydr. Polym. 2017, 157, 962–970.
- Nuruddin, M.; Hosur, M.; Uddin, M.J.; Baah, D.; Jeelani, S. A novel approach for extracting cellulose nanofibers from lignocellulosic biomass by ball milling combined with chemical treatment. J. Appl. Polym. Sci. 2016, 133, 42990–42999.
- Varanasi, S.; Henzel, L.; Sharman, S.; Batchelor, W.; Garnier, G. Producing nanofibres from carrots with a chemical-free process. Carbohydr. Polym. 2018, 184, 307–314.
- Yang, W.; Feng, Y.; He, H.; Yang, Z. Environmentally-friendly extraction of cellulose nanofibers from steam-explosion pretreated sugar beet pulp. Materials 2018, 11, 1160.
- Charoenrak, S.; Charumanee, S.; Sirisa-ard, P.; Bovonsombut, S.; Kumdhitiahutsawakul, L.; Kiatkarun, S.; Pathom-Aree, W.; Chitov, T.; Bovonsombut, S. Nanobacterial cellulose from Kombucha fermentation as a potential protective carrier of Lactobacillus plantarum under simulated gastrointestinal tract conditions. Polymers 2023, 15, 1356.
- Aditiawati, P.; Dungani, R.; Muharam, S.; Sulaeman, A.; Hartati, S.; Dewi, M.; Rosamah, E. The nanocellulose fibers from symbiotic culture of bacteria and yeast (SCOBY) kombucha: Preparation and characterization. In Nanofibers—Synthesis, Properties and Applications; Kumar, B., Ed.; IntechOpen: London, UK, 2021; Chapter 8; pp. 1–13.
- Dima, S.-O.; Panaitescu, D.-M.; Orban, C.; Ghiurea, M.; Doncea, S.-M.; Fierascu, R.C.; Nistor, C.L.; Alexandrescu, E.; Nicolae, C.-A.; Trică, B.; et al. Bacterial nanocellulose from side-streams of kombucha beverages production: Preparation and physical-chemical properties. Polymers 2017, 9, 374.
- Ullah, H.; Wahid, F.; Santos, H.A.; Khan, T. Advances in biomedical and pharmaceutical applications of functional bacterial cellulose-based nanocomposites. Carbohydr. Polym. 2016, 150, 330–352.
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose 2016, 23, 57–91.
- de Oliveira Barud, H.G.; da Silva, R.R.; da Silva Barud, H.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; de Oliveira, O.B., Jr.; Ribeiro, S.J.L. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohydr. Polym. 2016, 153, 406–420.
- Huang, Y.; Zhu, C.; Yang, J.; Nie, Y.; Chen, C.; Sun, D. Recent advances in bacterial cellulose. Cellulose 2014, 21, 1–30.
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent advances and applications of bacterial cellulose in biomedicine. Polymers 2021, 13, 412.
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Koloor, S.S.R.; Petrů, M. Micro- and nanocellulose in polymer composite materials: A review. Polymers 2021, 13, 231.
- Bilgi, E.; Bayir, E.; Sendemir-Urkmez, A.; Hames, E.E. Optimization of bacterial cellulose production by Gluconacetobacter xylinus using carob and haricot bean. Int. J. Biol. Macromol. 2016, 90, 2–10.
- Fernandes, I.A.A.; Pedro, A.C.; Ribeiro, V.R.; Bortolini, D.G.; Ozaki, M.S.C.; Maciel, G.M.; Haminiuk, C.W.I. Bacterial cellulose: From production optimization to new applications. Int. J. Biol. Macromol. 2020, 164, 2598–2611.
- Raiszadeh-Jahromi, Y.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Optimization of bacterial cellulose production by Komagataeibacter xylinus PTCC 1734 in a low-cost medium using optimal combined design. J. Food Sci. Technol. 2020, 57, 2524–2533.
- Revin, V.; Liyaskina, E.; Nazarkina, M.; Bogatyreva, A.; Shchankin, M. Cost-effective production of bacterial cellulose using acidic food industry by-products. Braz. J. Microbiol. 2018, 49S, 151–159.
- Yang, H.J.; Lee, T.; Kim, J.R.; Choi, Y.-E.; Park, C. Improved production of bacterial cellulose from waste glycerol through investigation of inhibitory effects of crude glycerol-derived compounds by Gluconacetobacter xylinus. J. Ind. Eng. Chem. 2019, 75, 158–163.
- Ye, J.; Zheng, S.; Zhang, Z.; Yang, F.; Ma, K.; Feng, Y.; Zheng, J.; Mao, D.; Yang, X. Bacterial cellulose production by Acetobacter xylinum ATCC 23767 using tobacco waste extract as culture medium. Bioresour. Technol. 2019, 274, 518–524.
- Dubey, S.; Singh, J.; Singh, R.P. Biotransformation of sweet lime pulp waste into high-quality nanocellulose with an excellent productivity using Komagataeibacter europaeus SGP37 under static intermittent fed-batch cultivation. Bioresour. Technol. 2018, 247, 73–80.
- Barshan, S.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Optimization and characterization of bacterial cellulose produced by Komagatacibacter xylinus PTCC 1734 using vinasse as a cheap cultivation medium. Int. J. Biol. Macromol. 2019, 136, 1188–1195.
