NCs include all cellulose-based particles (i.e., at least one dimension in tens of nanometers) having various shapes, sizes, surface chemistries and properties [
7]. Considering their sizes and functions, which in turn depend on the source and processing conditions, NCs can be classified into three main subcategories (
Table 1): short, rigid cellulose nanocrystals (CNCs); long flexible cellulose nanofibrils (CNFs); and highly crystalline pure bacterial nanocellulose (BNC) [
8,
9,
10,
11].
Table 1. Sources, preparation techniques and special characteristics of NCs.
Abbreviations: W—width; L—length.
These three types of NCs resemble each other by having a relatively close chemical compositions, but they clearly differ in particle size, degree of crystallinity and morphological properties [
26]. These specific characteristics, which distinguish them from one another, crucially depend on the source of origin and the processing technique of the materials. Thus, considering the essential importance of these two parameters, they are presented below, detailed for each type of nanocellulose.
2. Sources for Nanocellulose Materials
One of the main parameters that impact the special characteristics of cellulose nanomaterials, presented in
Table 1, is their original source [
27,
28]. There are four main sources of nanocellulose: plants (trees, shrubs and herbs), bacterial species (
Acetobacter,
Agrobacterium,
Alcaligenes,
Pseudomonas,
Rhizobium or
Sarcina), algae (
Phaeophyta,
Chlorophyta,
Rodophyta, etc.) and animals (Tunicata) [
29].
Cellulose nanocrystals (CNCs), also referred to as nanocrystalline cellulose, cellulose nanowhiskers or cellulose crystallites, are extracted from wood or non-woody biomass (agricultural residues and annual plants) by chemically removing (i.e., acid hydrolysis) the lignin, hemicellulose and the amorphous regions of cellulose [
31]. Compared to wood, the non-woody biomass contains low amounts of lignin and hemicelluloses, which allows easier access to the cellulose without the use of severe chemical treatments. Another source of CNCs extraction is industrial biowaste, a cheap source with low or even negative costs, which can be a solution to the current environmental problems regarding their elimination from industries [
28]. Tunicates are considered the only animal source of nanocellulose, and these are synthesized by some specific enzyme complexes from the plasma membrane of epidermal cells [
22]. Tunicate cellulose aggregates are composed of nearly pure cellulose Iβ allomorph [
32], and after acid hydrolysis, these yield long nanoparticles with a high aspect ratio, high crystallinity and good mechanical strength [
33,
34].
Cellulose nanofibrils (CNFs), also known as microfibrillated cellulose, microfibrils or nanofibrillated cellulose, are extracted from wood, sugar beet, potato tuber, hemp, or flax by delamination of the pulp through mechanical treatments (under high pressure), usually after enzymatic prehydrolysis or chemical treatments [
10]. CNFs can be biosynthesized in small quantities by brown (
Phaeophyta), green (
Chlorophyta), red (
Rhodophyta), blue–green (
Cynophyta) or golden algae (
Ochrophyta) [
35,
36,
37,
38], and their structure varies depending on the algae specie. Algae have the advantage of growing faster than terrestrial counterparts, and in addition, they have a low lignin content, which represents an advantage. Besides this, algae are obtained in large quantities as waste from agar production; thus, they can be considered an alternative source for the production of nanocellulose to meet future demands [
9].
Bacterial nanocellulose (BNC), also known as microbial cellulose or biocellulose, is a pure component of the biofilm resulting from the activity of aerobic bacteria, such as those belonging to the genus
Gluconacetobacter [
39].
3. Isolation Methods
Different isolation procedures are used to obtain NCs, depending on their source. These procedures are presented in detail below for each type of nanocellulose discussed: CNCs, CNFs and BNC.
3.1. CNCs
NCs in nanocrystalline form are one of the most studied types of nanocellulose in term of production methods and their properties [
29]. The common method of separating CNCs is controlled hydrolysis of cellulose using mineral acids [
12,
40,
41]. However, this method is part of a multi-step procedure that begins with alkali and bleaching pretreatments and is followed by acid hydrolysis, washing, centrifugation, dialysis, and ultrasonication to form a suspension that may be further subjected to lyophilization [
19]. The isolation of CNCs involves turning large pieces of starting material into fine nanoparticles. Thus, at the macroscopic or microscopic level, a transverse cleavage occurs along the amorphous regions of the cellulose, resulting in a rod-like material, as shown in
Figure 1 [
41,
42].
Figure 1. Scheme for obtaining CNCs by acid hydrolysis of cellulose, with the removal of the amorphous region and leaving only the crystalline region.
The main sources of CNCs and preparation conditions are presented in
Table 2. The isolation of CNCs involves the use of various acids such as sulfuric, hydrochloric, hydrobromic and phosphoric acid, but of these, sulfuric acid is overwhelmingly the most used [
29,
43,
44,
45]. The use of different acids produces CNCs with different properties, among which is notably the ability to disperse in aqueous medium, rheological behavior, morphology or crystallinity degree [
41]. Besides acid type, the reaction time is also an important parameter with great influence on the crystallinity degree. The longer the contact time, the greater the removal of the amorphous regions within the sample. However, a long contact time can degrade cellulose or even break it down into its precursor sugars. Considering the fact that short reaction times are not enough to obtain nanocrystals, it is obvious that it is necessary to find a balance in order to obtain the final product with the desired properties [
40,
44].
In the past few years, new routes have emerged regarding CNCs preparation, namely esterification using concentrated organic acids [
37], periodate oxidation [
13], TEMPO-mediated oxidation [
46], reductive amination [
47], and microbial or enzymatic hydrolysis [
32,
48]. The preparation conditions using these types of reactants are also presented in
Table 2.
Table 2. CNCs sources as well as the preparation techniques and conditions.
Abbreviations: PTA—Phosphotungstic acid; NaIO4—Sodium (meta) periodate; H3PO4—Phosphoric acid; H2SO4—Sulfuric acid; TEMPO—(2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl.
3.2. CNFs
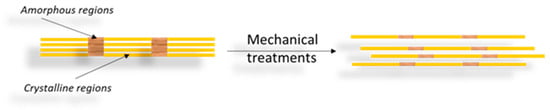
CNFs are the second type of nanocellulose and have a web-like network structure. These are commonly derivatives from natural sources such as wood pulp by mechanical processes (high-pressure homogenization, grinding and refining) preceded or followed by chemical or enzymatic treatments [
28,
42]. Therefore, the process of obtaining CNFs results in nanofibrils built up by alternating amorphous and crystalline regions (
Figure 2) whose cross-sections measure from tens to several hundreds of nanometers, while their length can reach several micrometers [
31,
40,
50].
Figure 2. Scheme for obtaining CNFs through a mechanical process of cleaving the cellulose fiber to the nanometric size.
High-pressure homogenization (HPH) is the most widely used technique for both the laboratory and industrial-scale production of CNFs. However, other strategies are also applied, such as micro-fluidization, micro-grinding, cryo-crushing and ultrasonication [
22,
31,
41]. In addition, different pre-treatments can be utilized before mechanical processes in order to reduce energy consumption as well as to make the surface hydrophobic, such as TEMPO oxidation [
51,
52], acetylation [
53], carboxymethylation [
54], alkali pretreatment [
55] and enzymatic pretreatment [
56]. For instance, an environmentally friendly process of preparing CNFs was reported by Mhlongo and coworkers [
57] using industrial hemp (
Cannabis sativa L.) bast fibers. The process combines the acid hydrolysis treatment with ultrasonication. Besides the fact that low-cost and sustainable industrial waste fibers are used, the obtained CNFs have superior crystallinity and thermal stability.
CNFs were first isolated in 1983 by Turbak and coworkers from bleached softwood fibers using high-pressure homogenization [
58]. Additionally, cellulose nanofibers have been obtained from pear [
59], Helicteresisora plant [
60], oil palm tree [
61], banana [
62,
63,
64,
65], Citrullus colocynthis seeds [
66], cassava peel [
67]; hemp [
68,
69], kenaf [
70], wheat straw [
71], bagasse [
56,
70], carrots [
72], etc. The main sources of CNFs and their preparation conditions are presented in
Table 3.
Table 3. CNFs sources, preparation techniques and conditions.
Abbreviations: SA—Sulfuric acid; FA—Formic acid; AA—acetic acid; MA—Maleic acid; PFA—Peroxyformic acid; PAA—peroxyacetic acid; TEMPO—2,2,6,6-tetramethylpiperidin-1-oxyl; ABS—acetate buffer solution.
3.3. BNC
BNC is produced by some bacteria in the form of an extracellular material, which is a direct response to their exposure to ultraviolet light, for example, or when defending against fungi, yeasts and other organisms [
22]. These bacteria are able to directly produce cellulose microfibrils through microbial fermentation, but without the hierarchical order found in plant cell walls [
43].
Some microorganisms with the ability to produce BNC have been reported, namely
Acetobacter (A.) xylinum,
Salmonella spp. and
Escherichia coli. In addition to these, nanocellulose fibers produced by the interaction of acetic acid bacteria and yeast through the kombucha fermentation process, known as Symbiotic Culture of Bacteria and Yeast (SCOBY), can also be mentioned here [
74,
75,
76]. SCOBY fibers have significant potential and are suitable for various applications (i.e., food, pharmaceutical, textiles, cosmetics) due to their special characteristics, such as strong gel film, high elasticity, and optimal deformation and comfort properties. For instance, SCOBY fibers are considered a potential substitute for cotton, a raw material for fabrics, because they have a flexible texture and are brown like synthetic leather. Moreover, they can be considered cheap and environmentally friendly fibers due to their high degradation rate in the environment [
75].
However, the bacterium
A. xylinum continues to be the highest producer of BNC so far [
77].
A. xylinum is unique in its family for being able to convert carbohydrates into acetic acid during bacterial growth and cellulose production [
42,
78]. These acetic acid bacteria secrete through their tiny pores located on the cell membrane an abundant 3D network of cellulose fibrils under aerobic conditions, using glucose as a carbon source [
79]. A single
A. xylinum cell may polymerize up to 200,000 glucose molecules per second, with cellulose synthase or terminal complexes presented in pores on the cell surface and then extruded into the surrounding medium [
80]. The cellulose excreted by
A. xylinum has a chemical structure identical to plant cellulose [
23], but it has the advantage of being pure cellulose and therefore does not require rigorous processing (i.e., chemical treatments) to eliminate undesired contaminants or impurities such as pectin, hemicellulose and lignin, as is the case with plant cellulose [
41].
Most bacterial cellulose is produced via the conventional static fermentation technique, whereby the bacteria can grow in shallow containers of semi-defined growth medium, in a static incubator at 30 °C, for 7 to 14 days. BNC accumulates at the air–liquid interface as a thick, leather-like, white pellicle that can be easily harvested from the liquid surface interface [
81]. Another fermentation technique is the dynamic one (with continuous stirring), in which BNC is obtained dispersed in the culture medium in the form of irregular pellets or suspended fibers [
82].
The production parameters, including temperature, pH, culture medium, inoculum ratio and incubation time, should be optimized using readily available and cheap raw materials for the production of high-quality and cost-effective BNC with high yield [
83]. Besides the selection of microorganisms and the fermentation method (static or dynamic), the choice of culture conditions has a high impact on BNC production [
84]. Studies have shown that up to 30% of the cost of the fermentation bioprocess is due to the fermentation/culture medium, whose composition (i.e., glucose, fructose, etc.) influences the efficiency of bacterial nanocellulose production. Therefore, a high concentration of sugars is necessary for the culture media to improve productivity, which ultimately increases the overall bioprocess cost [
85]. Residual products from the dairy industry, wheat straw, fruit juices, rotten fruit, molasses, wine fermentation broth and others have also been used as a source of nutrients for low-cost BNC production [
86]. Thus, several researchers have evaluated new sources of carbon and cultivation conditions to optimize BNC production and achieve a more significant yield with reduced costs and production time, and some of the results obtained are presented in
Table 4.
Table 4. Optimization of BNC production using different bacteria strains, fermentation techniques and carbon sources.
|
Bacteria Strain
|
Fermentation
Technique
|
Carbon Source
|
Optimal Fermentation
Conditions
|
Productivity, g/L/day
|
Ref.
|
|
G. xylinus
ATCC 700178
|
Static
|
Carob/Haricot bean
|
2.5 g/L carbon, T = 30 °C,
pH = 5.5, t = 9 days
|
0.19
|
[83]
|
|
G. xylinus KCCM 41431
|
Static
|
Residual crude glycerol
|
20 g/L glycerol, pH = 5,
t = 7 days
|
0.99
|
[87]
|
|
G. xylinus
PTCC 1734
|
Static
|
Beet molasses/Cheese whey
|
T = 28 °C, pH = 5.5,
t = 14 days
|
0.32
|
[18]
|
|
G. sucrofermentans B-11267
|
Dynamic
|
Wheat vinasse
/Cheese whey
|
T = 28 °C, pH = 3.95–4.96,
t = 3 days, 250 rpm
|
2.06
|
[86]
|
|
A. xylinum ATCC 23767
|
Static
|
Waste extract tobacco
|
T = 30 °C, pH = 6.5,
t = 7 days
|
0.32
|
[88]
|
|
Dynamic
|
T = 30 °C, 150 rpm
|
0.74
|
|
K. europaeus SGP37
|
Static batch/Static intermittent fed-batch
|
Sweet lime pulp waste
|
T = 30 °C, pH = 6, t = 16 days;
Addition every 48 h and 96 h
|
0.40
|
[89]
|
|
K. xylinus
PTCC 1734
|
Static
|
Vinasse
|
40% vinasse, T = 30 °C,
pH = 6, t = 10 days
|
0.18
|
[90]
|
|
K. xylinus
PTCC 1734
|
Dynamic
|
Date syrup/Cheese whey
|
Date syrup: cheese whey ratio = 50:50, T = 28 °C, pH = 4.48, t = 10 days
|
0.19
|
[85]
|
Abbreviations: G. xylinus—Gluconacetobacter xylinus; A. xylinum—Acetobacter xylinum; K. xylinus—Komagatacibacter xylinus; K. europaeus—Komagataeibacter europaeus.