Integrins are heterodimeric transmembrane proteins that mediate adhesive connections between cells and their surroundings, including surrounding cells and the extracellular matrix (ECM). They modulate tissue mechanics and regulate intracellular signaling, including cell generation, survival, proliferation, and differentiation, and the up-regulation of integrins in tumor cells has been confirmed to be associated with tumor development, invasion, angiogenesis, metastasis, and therapeutic resistance. Thus, integrins are expected to be an effective target to improve the efficacy of tumor therapy. A variety of integrin-targeting nanodrugs have been developed to improve the distribution and penetration of drugs in tumors, thereby, improving the efficiency of clinical tumor diagnosis and treatment.
- integrin
- cell adhesion
- cytoskeleton
- tumor
1. The Structure of Integrins
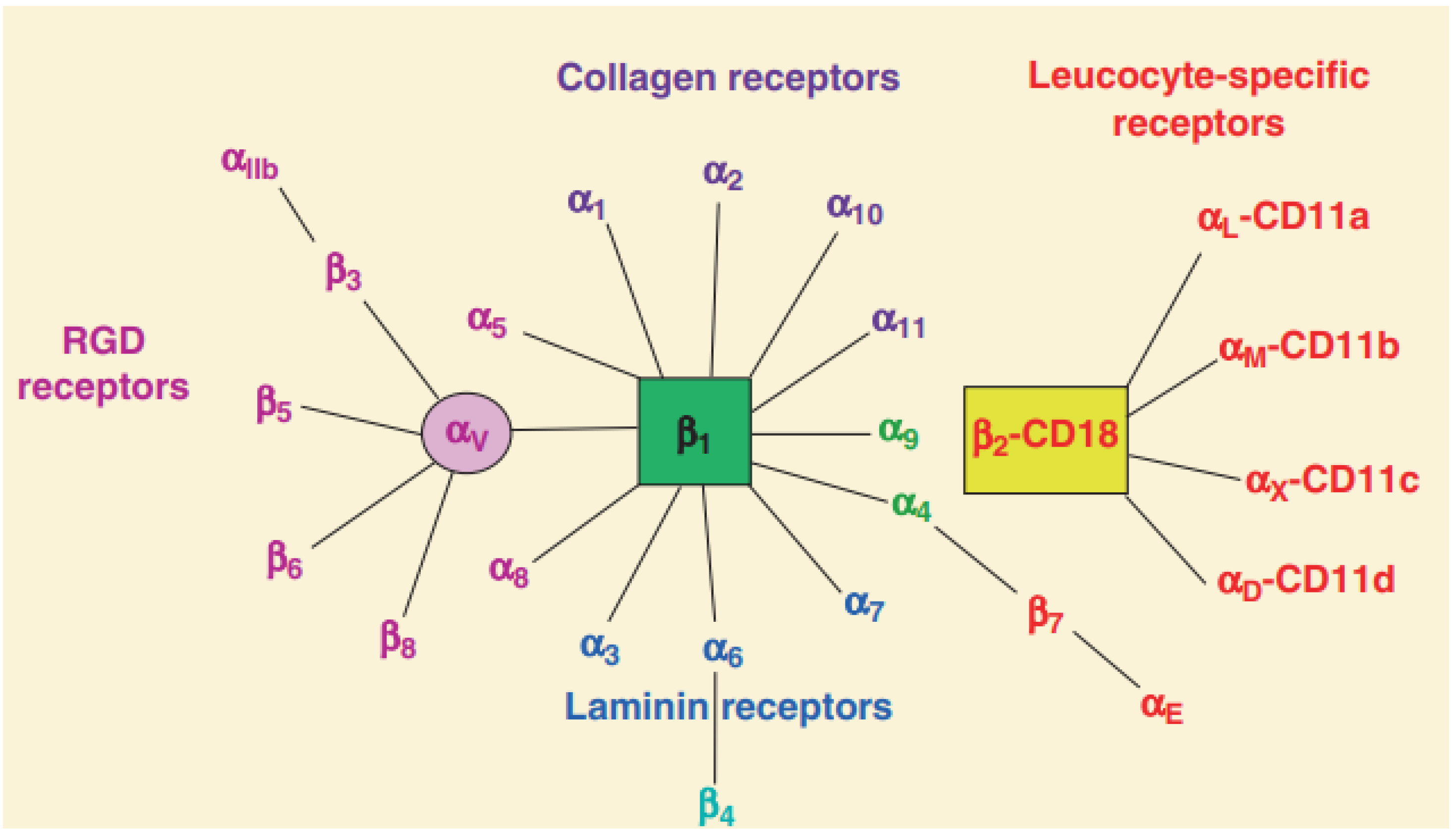
2. The Function of Integrins
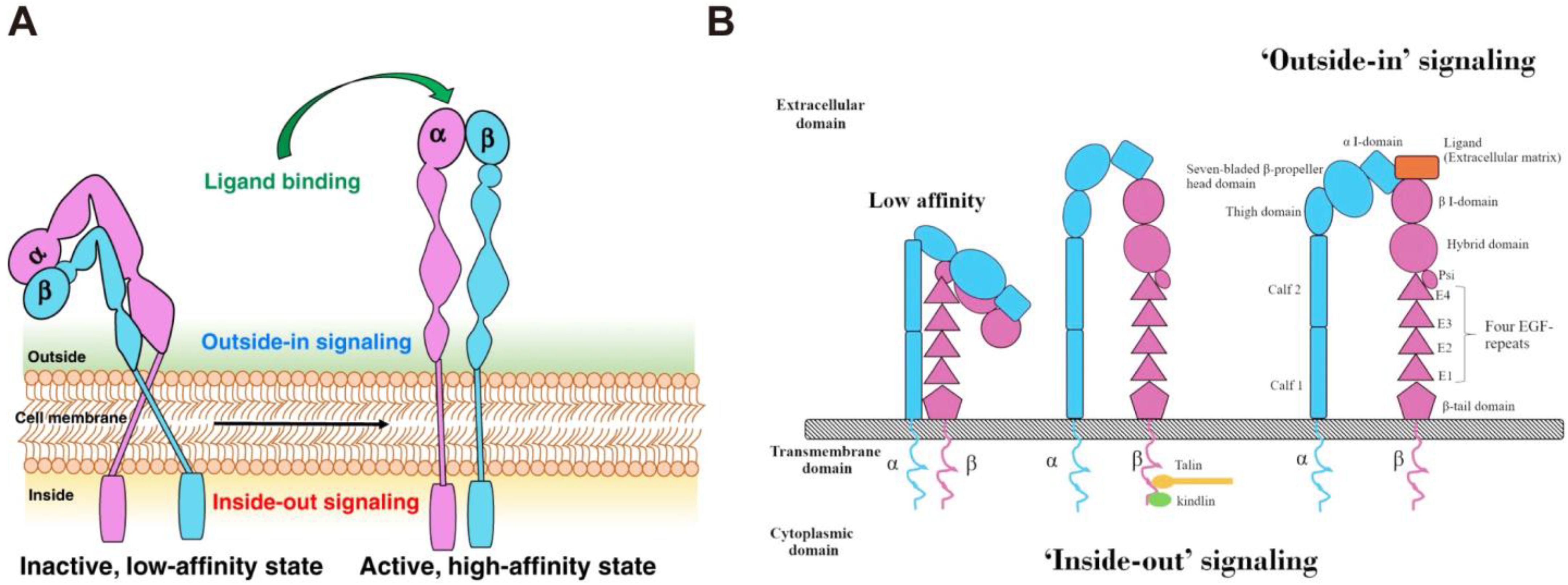
3. The Effect of Integrins on Tumors
This entry is adapted from the peer-reviewed paper 10.3390/nano13111721
References
- Berman, A.E.; Kozlova, N.I. Integrins: Structure and Functions. Membr. Cell Biol. 2000, 13, 207–244.
- Niu, G.; Chen, X. Why Integrin as a Primary Target for Imaging and Therapy. Theranostics 2011, 1, 30–47.
- Takada, Y.; Ye, X.; Simon, S. The Integrins. Genome Biol. 2007, 8, 215.
- Shi, J.; Wang, F.; Liu, S. Radiolabeled Cyclic RGD Peptides as Radiotracers for Tumor Imaging. Biophys. Rep. 2016, 2, 1–20.
- Shimaoka, M.; Springer, T.A. Therapeutic Antagonists and Conformational Regulation of Integrin Function. Nat. Rev. Drug Discov. 2003, 2, 703–716.
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280.
- Humphries, M.J.; Symonds, E.J.; Mould, A.P. Mapping Functional Residues onto Integrin Crystal Structures. Curr. Opin. Struct. Biol. 2003, 13, 236–243.
- Arimori, T.; Miyazaki, N.; Mihara, E.; Takizawa, M.; Taniguchi, Y.; Cabañas, C.; Sekiguchi, K.; Takagi, J. Structural Mechanism of Laminin Recognition by Integrin. Nat. Commun. 2021, 12, 4012.
- Pan, L.; Zhao, Y.; Yuan, Z.; Qin, G. Research Advances on Structure and Biological Functions of Integrins. SpringerPlus 2016, 5, 1094.
- Kolasangiani, R.; Bidone, T.C.; Schwartz, M.A. Integrin Conformational Dynamics and Mechanotransduction. Cells 2022, 11, 3584.
- Tong, D.; Soley, N.; Kolasangiani, R.; Schwartz, M.A.; Bidone, T.C. Integrin AIIbβ3 Intermediates: From Molecular Dynamics to Adhesion Assembly. Biophys. J. 2023, 122, 533–543.
- Assa-Munt, N.; Jia, X.; Laakkonen, P.; Ruoslahti, E. Solution Structures and Integrin Binding Activities of an RGD Peptide with Two Isomers. Biochemistry 2001, 40, 2373–2378.
- van der Flier, A.; Sonnenberg, A. Function and Interactions of Integrins. Cell Tissue Res. 2001, 305, 285–298.
- Hood, J.D.; Cheresh, D.A. Role of Integrins in Cell Invasion and Migration. Nat. Rev. Cancer 2002, 2, 91–100.
- Vasconcelos, A.A.; Estrada, J.C.; David, V.; Wermelinger, L.S.; Almeida, F.C.L.; Zingali, R.B. Structure-Function Relationship of the Disintegrin Family: Sequence Signature and Integrin Interaction. Front. Mol. Biosci. 2021, 8, 1178.
- Su, Y.; Iacob, R.E.; Li, J.; Engen, J.R.; Springer, T.A. Dynamics of Integrin α5β1, Fibronectin, and Their Complex Reveal Sites of Interaction and Conformational Change. J. Biol. Chem. 2022, 298, 102323.
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting Integrin Pathways: Mechanisms and Advances in Therapy. Sig. Transduct. Target. Ther. 2023, 8, 1.
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The Role of Integrins in Inflammation and Angiogenesis. Pediatr. Res. 2021, 89, 1619–1626.
- Thomas Parsons, J.; Slack-Davis, J.K.; Tilghman, R.W.; Iwanicki, M.; Martin, K.H. Chapter 66—Integrin Signaling: Cell Migration, Proliferation, and Survival. In Handbook of Cell Signaling, 2nd ed.; Bradshaw, R.A., Dennis, E.A., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 491–499. ISBN 978-0-12-374145-5.
- Takagi, J.; Springer, T.A. Integrin Activation and Structural Rearrangement. Immunol. Rev. 2002, 186, 141–163.
- Kim, M.; Carman, C.V.; Springer, T.A. Bidirectional Transmembrane Signaling by Cytoplasmic Domain Separation in Integrins. Science 2003, 301, 1720–1725.
- Kanchanawong, P.; Calderwood, D.A. Organization, Dynamics and Mechanoregulation of Integrin-Mediated Cell-ECM Adhesions. Nat. Rev. Mol. Cell Biol. 2023, 24, 142–161.
- Kulke, M.; Langel, W. Molecular Dynamics Simulations to the Bidirectional Adhesion Signaling Pathway of Integrin αVβ3. Proteins Struct. Funct. Bioinform. 2020, 88, 679–688.
- Calderwood, D.A.; Campbell, I.D.; Critchley, D.R. Talins and Kindlins: Partners in Integrin-Mediated Adhesion. Nat. Rev. Mol. Cell Biol. 2013, 14, 503–517.
- Bouti, P.; Webbers, S.D.S.; Fagerholm, S.C.; Alon, R.; Moser, M.; Matlung, H.L.; Kuijpers, T.W. β2 Integrin Signaling Cascade in Neutrophils: More Than a Single Function. Front. Immunol. 2021, 11, 619925.
- Ren, D.; Zhao, J.; Sun, Y.; Li, D.; Meng, Z.; Wang, B.; Fan, P.; Liu, Z.; Jin, X.; Wu, H. Overexpressed ITGA2 Promotes Malignant Tumor Aggression by Up-Regulating PD-L1 Expression through the Activation of the STAT3 Signaling Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 485.
- Guo, P.; Moses-Gardner, A.; Huang, J.; Smith, E.R.; Moses, M.A. ITGA2 as a Potential Nanotherapeutic Target for Glioblastoma. Sci. Rep. 2019, 9, 6195.
- Xiong, J.; Yan, L.; Zou, C.; Wang, K.; Chen, M.; Xu, B.; Zhou, Z.; Zhang, D. Integrins Regulate Stemness in Solid Tumor: An Emerging Therapeutic Target. J. Hematol. Oncol. 2021, 14, 177.
- Rattanasinchai, C.; Navasumrit, P.; Ruchirawat, M. Elevated ITGA2 Expression Promotes Collagen Type I-Induced Clonogenic Growth of Intrahepatic Cholangiocarcinoma. Sci. Rep. 2022, 12, 22429.
- Slack, R.J.; Macdonald, S.J.F.; Roper, J.A.; Jenkins, R.G.; Hatley, R.J.D. Emerging Therapeutic Opportunities for Integrin Inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78.
- Niu, J.; Li, Z. The Roles of Integrin Avβ6 in Cancer. Cancer Lett. 2017, 403, 128–137.
- Wei, L.; Zhou, Q.; Tian, H.; Su, Y.; Fu, G.-H.; Sun, T. Integrin β3 Promotes Cardiomyocyte Proliferation and Attenuates Hypoxia-Induced Apoptosis via Regulating the PTEN/Akt/MTOR and ERK1/2 Pathways. Int. J. Biol. Sci. 2020, 16, 644–654.
- Aksorn, N.; Chanvorachote, P. Integrin as a Molecular Target for Anti-Cancer Approaches in Lung Cancer. Anticancer Res. 2019, 39, 541–548.
- Hamidi, H.; Ivaska, J. Every Step of the Way: Integrins in Cancer Progression and Metastasis. Nat. Rev. Cancer 2018, 18, 533–548.
- Li, M.; Wang, Y.; Li, M.; Wu, X.; Setrerrahmane, S.; Xu, H. Integrins as Attractive Targets for Cancer Therapeutics. Acta. Pharm. Sin. B 2021, 11, 2726–2737.
- Bergonzini, C.; Kroese, K.; Zweemer, A.J.M.; Danen, E.H.J. Targeting Integrins for Cancer Therapy—Disappointments and Opportunities. Front. Cell Dev. Biol. 2022, 10, 863850.
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160.
- Li, X.; Sun, X.; Kan, C.; Chen, B.; Qu, N.; Hou, N.; Liu, Y.; Han, F. COL1A1: A Novel Oncogenic Gene and Therapeutic Target in Malignancies. Pathol. Res. Pract. 2022, 236, 154013.
- Baltes, F.; Pfeifer, V.; Silbermann, K.; Caspers, J.; Wantoch von Rekowski, K.; Schlesinger, M.; Bendas, G. β1-Integrin Binding to Collagen Type 1 Transmits Breast Cancer Cells into Chemoresistance by Activating ABC Efflux Transporters. Biochim. Biophys. Acta. Mol. Cell Res. 2020, 1867, 118663.
- Reis, J.S.D.; Santos, M.A.R.d.C.; da Costa, K.M.; Freire-de-Lima, C.G.; Morrot, A.; Previato, J.O.; Previato, L.M.; da Fonseca, L.M.; Freire-de-Lima, L. Increased Expression of the Pathological O-Glycosylated Form of Oncofetal Fibronectin in the Multidrug Resistance Phenotype of Cancer Cells. Matrix Biol. 2023, 118, 47–68.
- Majidpoor, J.; Mortezaee, K. Steps in Metastasis: An Updated Review. Med. Oncol. 2021, 38, 3.
- Zhang, Y.; Qian, J.; Gu, C.; Yang, Y. Alternative Splicing and Cancer: A Systematic Review. Signal. Transduct. Target. Ther. 2021, 6, 78.
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674.
- Valdembri, D.; Serini, G. The Roles of Integrins in Cancer. Fac. Rev. 2021, 10, 45.
- Moschos, S.J.; Drogowski, L.M.; Reppert, S.L.; Kirkwood, J.M. Integrins and Cancer. Oncology 2007, 21, 13–20.
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in Cancer: Biological Implications and Therapeutic Opportunities. Nat. Rev. Cancer 2010, 10, 9–22.
- Cordes, N.; Park, C.C. Beta1 Integrin as a Molecular Therapeutic Target. Int. J. Radiat. Biol. 2007, 83, 753–760.
- Blandin, A.-F.; Renner, G.; Lehmann, M.; Lelong-Rebel, I.; Martin, S.; Dontenwill, M. β1 Integrins as Therapeutic Targets to Disrupt Hallmarks of Cancer. Front. Pharmacol. 2015, 6, 279.
- Hou, J.; Yan, D.; Liu, Y.; Huang, P.; Cui, H. The Roles of Integrin α5β1 in Human Cancer. Onco Targets Ther. 2020, 13, 13329–13344.
- Fang, Z.; Yao, W.; Fu, Y.; Wang, L.-Y.; Li, Z.; Yang, Y.; Shi, Y.; Qiu, S.; Fan, J.; Zha, X. Increased Integrin A5β1 Heterodimer Formation and Reduced C-Jun Expression Are Involved in Integrin β1 Overexpression-Mediated Cell Growth Arrest. J. Cell. Biochem. 2010, 109, 383–395.
