1. Introduction
In addition to, or alternatively to, decarboxylation some
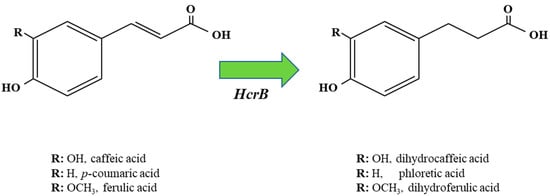
Lactobacillus spp. display the capacity to reduce hydroxycinnamic acids into phenylpropionic acids [
17,
34] (
Figure 4). Among lactobacilli, the enzymes involved in this reduction process have been solely validated from a biochemical viewpoint in
L. plantarum, and are encoded by the
hcrA and
hcrB genes. Whole-genome transcriptional profiling studies have shown that the expression levels of the
hcrAB genes are strongly elevated in the presence of
p-CA [
8]. The
hrcB transcripts were also overexpressed in pineapple juices [
35], a plant food matrix rich in hydroxycinamic acids [
36]. The
L. plantarum hydroxycinamate reductase (HCR) proteins (HcrAB) encoded by the
hcrAB genes have been recently purified and characterized [
37]. The characterization of the HCR activity by these enzymes has been mainly performed by using
m-coumaric acid as a model HCA, as
L. plantarum does not metabolize this compound by decarboxylation but only by reduction to 3-(3)-hydroxyphenyl)propionic acid (3-HPPA) [
34]. It was determined that HcrA did not exhibit HCR catalytic activity in vitro in the presence of the required FMN and NADH cofactors. However, this protein improved HcrB production when both enzymes were coproduced together. In addition, the coproduction of these enzymes reduced the partial proteolysis of HcrB (and its subsequent partial inactivation), observed when this protein was purified alone [
37]. It was then proposed that the role of HcrA is to stabilize and facilitate the solubility of the heterodimer that this protein forms with HcrB [
37], similarly to other coupled FMN reductases described in
Lactobacillus acidophilus [
38] and
Lactobacillus johnsonii [
39]. However, up to now it is unknown if the NAD(P)H-binding domain (COG0431) present in HcrA serves to bind a nicotinamide cofactor and if it participates in the electron transfer on the way to the final acceptor by the HcrAB heterodimer, as it occurs with the COG0431 domain of the contiguous HcrB, an enzyme that has been shown to require and bind NADH for activity.
Figure 4. Graphic representation of metabolic reduction of caffeic, p-coumaric and ferulic acid into phenylpropionic derivatives by Lactobacillus spp. HcrB, hydroxycinnamate reductase B. Each phenylpropionic acid shares the same R group as the hydroxycinnamic acid from which it is derived.
By using extracts from
Escherichia coli overexpressing the recombinant HcrB protein, instead of using the purified HcrB protein, it was possible to relieve its partial hydrolysis and inactivation and determine that HcrB (EC.1.3.1.11) constituted the minimum requirement for HCR activity [
36]. Thus, the HcrB protein was demonstrated to act on the unsaturated bond of the ethylenic side chain of HCAs to reduce (although only partially) the
p-coumaric,
m-coumaric,
o-coumaric, ferulic, caffeic and sinapic acids into their corresponding phenylpropionic acid derivatives [
37].
In spite of the presence of the double bond in the aliphatic side chain of HCAs possibly being expected to provide greater H-donating ability and subsequent radical stabilization, some phenylpropionic acid derivatives, such as dihydrocaffeic acid, seem to confer a slightly improved resistance to oxidative stress compared to caffeic acid [
40], and show a radical scavenging activity higher than caffeic acid [
41]. However, this property is dependent on the type of HCA [
42] and the system where the oxidation happens [
40]. Therefore, it is not clear whether the reduction of HCAs into HPPA derivatives provides an advantage and significantly increases the antioxidant efficiency of HCAs in lactobacilli.
Similarly to the decarboxylation process, the expression of the
hcrAB genes and hence the reduction process, is controlled by HcrR, a Lys-R type transcriptional regulator. The
hcrR gene lies downstream of
hcrB and is cotranscribed with
hcrA and
hrcB. The HcrR regulator participates and plays a key role in the reduction process, as shown by the absence of hydroxycinnamate reductase activity in a
L. plantarum WCFS1
hcrR knockout mutant [
37].
2. Variability in the HCA Reduction and Decarboxylation Metabolisms across Lactobacillus spp.
As mentioned above, it has been recently reported that the genetic complement for the metabolism of HCAs seems to be rather variable in
Lactobacillus spp. [
17]. Thus, while some of these bacteria apparently possess the complete gene toolkit required to metabolize HCAs via decarboxylation (
pad,
vprA) and reduction (
hcr) pathways, others encode PAD but not HCR enzymes, while others lack PAD but encode HCR and a few hold PAD and VprA while others encode VprA but not PAD [
17]. However, regarding the HCR enzymes, it must be reiterated that not all proposed putative enzymes have been biochemically validated to support their involvement in HCA reduction.
This comparative genomic analysis has identified homologs with different degrees of identity to
hrcA or
hrcB in heterofermentative lactobacilli. Some of the found
hrcB-like genes have been proposed as novel putative phenolic acid reductases on the basis that they were variably overexpressed in the presence of different hydroxycinnamic acids (sinapic, ferulic or caffeic acids) [
17]. One of these
hrcB-like genes, named
par1, was found in
F. rossiae strains that lack HrcB and displayed the lowest degree of identity to
L. plantarum WCFS 1 HcrB among the proposed putative HCR reductases. Interestingly, the deletion of
par1 rendered
F. rossiae mutants unable to reduce the mentioned acids to their corresponding phenylpropionic acids, showing its involvement in the reduction process in this strain [
17].
However, a transcriptomic study showed that
par1 homologs from
L. plantarum WCFS1 (
lp_0952,
lp_0055) are not responsive to
p-CA (in contrast to
hrcB) [
8]. In addition,
par1 homologs from other two
L. plantarum strains (TMW 1.460 and FUA3584) were also not responsive to other HCAs (sinapic, ferulic or caffeic acids) [
16]. These observations suggest that the sole presence of some
hrc or
hcr-like genes do not necessarily match with the reduction phenotype. It must also be noted that even the overexpression of these genes is not necessarily related to the reduction phenotype, as was shown in the case of cinnamic acid, a phenolic compound able to induce the expression of the
L. plantarum HcrB, but not to act as a substrate for this enzyme [
37]. Therefore, regarding HCR reductases, the genotype (presence or absence of the coding gene) and overexpression may not precisely forecast the phenotype. In this line, mutant approaches and biochemical characterization for other previously proposed putative reductases [
17] are required in order to validate their contribution to the reduction process. In addition, it would be necessary to continue the biochemical characterization of confirmed HCR reductases, such as Par1, to further confirm their implication in the reduction process and bring new information on the scarcely studied oxidoreductases that act on carbon–carbon double bonds of HCAs.
In view of the variability of HCA metabolism across lactobacilli, the observed differences in the genetic toolkits for HCA metabolism among species and the high variability in the expression profile of these metabolic genes, new studies will probably be necessary to validate and ascribe a role to the proposed putative enzymes in the metabolism of HCAs.
3. HrcAB Reductase: Open Questions
HcrAB has been demonstrated to reduce HCAs; however, whole-genome transcription profiling revealed that
hcrAB genes were significantly upregulated in the presence of the phenolic compounds oleuropein (OLE) [
43] or gallic acid (GA) [
44]. This induction is significant, not least since the
hrcAB genes showed the highest induction among all the reductases whose expression were modulated by OLE. In the case of GA, HcrAB was the sole reductase induced in the presence of this hydroxybenzoic acid. Due to the structural differences between hydroxycinnamic acids, OLE and GA, the observed expression profiles provide hints that the activity of HcrAB phenolic reductase could be not restricted to hydroxycinnamic acids. Of note, it has been also observed that cinnammic acid also transcriptionally induces
hcrB [
37], albeit this acid does not apparently act as a substrate under the conditions assayed.
In addition, it is important to note that the current data on the transformation of HCAs into substituted phenyl-propionic acids by the
L. plantarum HcrB have been obtained from
E. coli extracts overexpressing this protein, as the pure HcrB protein, as mentioned above, underwent partial hydrolysis and substantially lost activity in the presence of oxygen [
37]. This low activity is in agreement with previous observations reporting that the reductase activity on HCAs is about 100 times lower than the decarboxylase activity [
19]. Altogether these results suggest that HcrB could be oxygen-sensitive, resembling strict oxygen-sensitive enoate reductases from
Clostridium acetobutylicum that are able to aerobically reduce cinnamic acid and
p-CA into 3-PPA and HPPA, respectively, in
E. coli [
45]. It has been proposed that the oxygen toxicity in the intracellular environment of
E. coli is low due to the expression of cellular respiratory chain on the membrane or enzymes such as SOD [
46,
47], which prevents the inactivation of the oxygen sensitive enzymes. Extracts from
E. coli that overexpress the
L. plantarum HcrB contain SOD, an enzyme able to prevent the oxygen toxicity of superoxide radicals which, however, probably cannot be relieved in the pure HcrB enzyme preparation.
Furthermore, it should not be forgotten that reductions mediated by HcrB, which display NAD(P)H and flavin-binding domains, have been shown to require NADH and FMN as cofactors [
36], which implies intramolecular electron transfer from NADH to the enzyme bound flavin FMN cofactor on the way to the final acceptor(s), the phenolic compound(s). The presence of oxygen, which is the best terminal electron acceptor in nature due to its high electronegativity (standard reduction potential of +1229 mV), can interfere with the electron transfer in the HcrB-catalyzed reaction and increase the rate of oxidation of HCAs, especially in the presence of traces of catalytic ubiquitous redox metals. In addition, the presence of oxygen can boost the competition for the cofactors required for the HcrB (NADH, FMN) by very competitive enzymes for NADH, particularly the NADH-oxidase [
48,
49], which could also explain the low prevalence of the reductase activity with respect to the decarboxylation in
L. plantarum, at least under aerobic conditions.
Considering that in L. plantarum, (i) the partial HcrB hydrolysis, (ii) the low HcrB activity, (iii) the potential competition of other enzymes for the cofactors NADH and FMN and (iv) the much lower HCR activity compared to decarboxylase activity are all fostered under aerobic conditions, a physiological advantage of using HcrB in the presence of oxygen appears unlikely. Therefore, assays and characterization of HrcB and other putative reductases from lactobacilli under anaerobic conditions could provide valuable new information on the mechanism of action and the range of substrates that these enzymes can reduce.
In fact, the physiological importance of the phenolic acid reductase activity has been investigated in heterofermentative lactic acid bacteria [
50,
51] under anaerobic conditions in order to exclude other final acceptors, such as the oxygen which is plentiful in the environment or fructose which is a major carbohydrate for some of these bacteria. Under these conditions, phenolic acids have been shown to be used as external electron acceptors. The physiological advantages of this strategy include an increase in the oxidation of NADH (increase in the NAD+/NADH ratio) accompanied by an increase in acetate production with the concomitant accumulation of additional ATP.
This entry is adapted from the peer-reviewed paper 10.3390/antiox12061294