Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Changes in fire regimes in the 21st century are posing a major threat to global biodiversity. In this scenario, incorporating species’ physiological, ecological, and evolutionary traits with their local fire exposure might facilitate accurate identification of species most at risk from fire.
- fire ecology
- resilience
- sensitivity
- functional traits
- savanna ecosystems
- species vulnerability
- fauna
- fire exposure
1. Introduction
Natural fire has shaped species evolution in savanna ecosystems worldwide [1][2]. In these ecosystems, animal species are relatively tolerant to low-severity and patchy fires. Natural fires usually allow individuals to survive or reestablish populations from adjacent preserved ecosystems after burning. However, extreme wildfires have resulted from a synergy between severe droughts, high temperatures, low air humidity, windy days, and increased human ignitions, which increase the flammability of terrestrial ecosystems and expand fire’s niche around the world [3][4][5][6]. Recent studies show alarming prospects, suggesting that under different future climate scenarios, changes in fire regimes are expected in the 21st century in terms of a meaningful increase, variability, and frequency of extreme events posing a major threat to global biodiversity [6][7].
2. Animal Functional Traits Associated with Fire Sensitivity
Researchers compiled 14 species traits that can increase the vulnerability of the species to the direct and indirect effects of fire. These traits are explained in detail below and are summarized in Table 1.
Table 1. Animal functional traits associated with fire sensitivity or fire resistance/resilience.
| Trait Group | Increase Vulnerability | Decrease Vulnerability | |
|---|---|---|---|
| DURING FIRE | Dormancy | Species that often express deep torpor on flammable surfaces in the flame zone. | Non-hibernators; species that rapidly arouse from shallow torpor when exposed to smoke or flame noises; species that remain in torpor in places protected from fire, such as in deeper soil layers |
| Escape decision | Animals that run away randomly when frightened; fossorial species with shallow burrowing behavior; species that take shelter in flammable or suffocating places, such as plants in the lower layers, litter, or cavities in small trees. | Animals that run toward nearby refuges when frightened; fossorial species with deep burrowing behavior; scansorial animals that seek refuge on top of tall trees during surface fires, in water, in termite mounds, or on rocky surfaces with little flammable material. | |
| Habitat use | Leaf litter-dwelling fauna in the o-horizon and other species that live or build nests in the lower strata of vegetation on flammable substrates, such as shrubs, grasses, dry and/or fallen trunks and branches, and small trees. | Soil-dwelling species that can burrow deeper into the ground; species that live or build nests close to perennial wetlands or water sources (semi-aquatic habits), below-ground, on rocky substrates, termite mounds with low flammability, and deep cavities inside massive tree trunks or in the upper strata of vegetation (on the top of tall trees). | |
| Mobility | Limited movement capability: slow-moving animals, weak flyers, ground-dwelling species that fail to climb trees, smaller jumpers with reduced effective jump height. | Good or excellent movement capability: fast runners, strong flyers, skilled climbers, larger jumpers with great effective jump height, and other jumping specialists that use catapult mechanisms. | |
| Morphology | Medium-bodied animals that may have difficulty fleeing or finding refuge; species whose bodies are covered with long, coarse fur or feathers. | Small-bodied animals that can find refuge more easily during a fire, while larger ones can flee or move away from affected areas; species with short fur, smooth skin, or covered with scales. | |
| Nest substrate | Species using flammable materials to build nests: thatched mounds, moss and lichen, fine grass or mammalian hair, and plant material such as bark, fiber, leaves, twigs, grasses, tussocks, and branches. | Species that use thermally insulating building materials: great amounts of soil in hard, protective clay mounds; species with deliberate behavior for modifying their surrounding environment causally reducing flammability; species that build subterranean nests without thatched mounds. | |
| Reproductive cycles | Synchronous reproduction, usually at the end of the dry season, exposing fragile life stages, pregnant, lactating, nesting, and brooding females to high-intensity fire. | Year-round breeders or species that reproduce during the wet season but decrease reproduction during the dry season. | |
| Sensory detection of fire cues | Species that spend most of their time in complex vegetation and rely primarily on the visual detection of fire (small-bodied animals could be even more vulnerable, as they usually have lower visual acuity). | Species that are able to detect olfactory and/or acoustic fire cues; species that can detect fire cues at lower thresholds; species that have thermoreceptors that can detect infrared radiation from fires; species relying primarily on the visual detection of fire, but that spend most of their time in the top of tall trees or open, low-stature vegetation and topographically simple landscapes. | |
| Social organization | Solitary animals or those that live in small family groups (parents and young); species with poorly developed social relationships (e.g., groups with weak connections) and whose individuals or groups lack effective communication skills. | Gregarious animals living in large groups; social species or those residing in more connected, reciprocal, and socially homogeneous groups. | |
| Behavioral plasticity | Late-successional species that require more structured habitats for nest sites and foraging, which take several years to recover. | Generalists that can temporarily adapt their diet and/or habitat preferences to the conditions and food resources available across the post-fire landscape; species that may benefit from fire-induced changes include early or mid-successional species. | |
| POST-FIRE | Dormancy | Species that express multi-day torpor but need to rewarm frequently; species that use daily torpor, which is not as deep as hibernation, lasts only some hours rather than days or weeks, and is usually—but not always—interrupted by daily foraging and feeding. | Invertebrates that remain inactive after a fire, allowing their tissues to become desiccated (anhydrobiosis); invertebrates that express aestivation and remain in an inactive stage remarkably resistant to water loss; species that use multi-day torpor for weeks or even months after a fire or during fire season without the need to rewarm. |
| Endogenous circadian rhythms | Diurnal ectotherms that depend on thermoregulation opportunities afforded by habitat structure; strictly diurnal prey species. | Nocturnal or crepuscular species; cathemeral or diurnal prey that can adjust their daily activity patterns. | |
| Mobility | Species with restricted home range; species with high site fidelity or territorial species; Migratory species (highly mobile), but with strong site fidelity. | Highly mobile species that travel long distances or show metapopulation dynamics; species with low site fidelity or non-territorial species. | |
| Morphology | Large ectotherms; invertebrates with thinner cuticles. | Large mammals; species capable of camouflaging in the scorched substrate; invertebrates with higher cuticle thickness. |
2.1. During Fire
High environmental temperatures predispose animals to heat stress, including physiological and behavioral disturbances such as hyperventilation and loss of coordination. For any species, there is a body temperature threshold beyond which cells undergo denaturation of proteins and membrane structures degrade, causing the individual’s death [8][9]. Beyond the heat from flames, reduced oxygen and exposure to toxic compounds following smoke inhalation may be critical factors that increase animal mortality during a fire [10]. The longer an animal is exposed to high temperatures, anoxia, or smoke inhalation, the greater the chances of mortality; therefore, detecting and avoiding fire are essential behaviors for survival, especially for less mobile animals [11]. It is also important to mention the increased vulnerability of prey to opportunistic predators while trying to escape fires. The physical and behavioral traits that can increase or decrease a species’ sensitivity to the direct effects of a fire are described in detail below and illustrated in Figure 1.
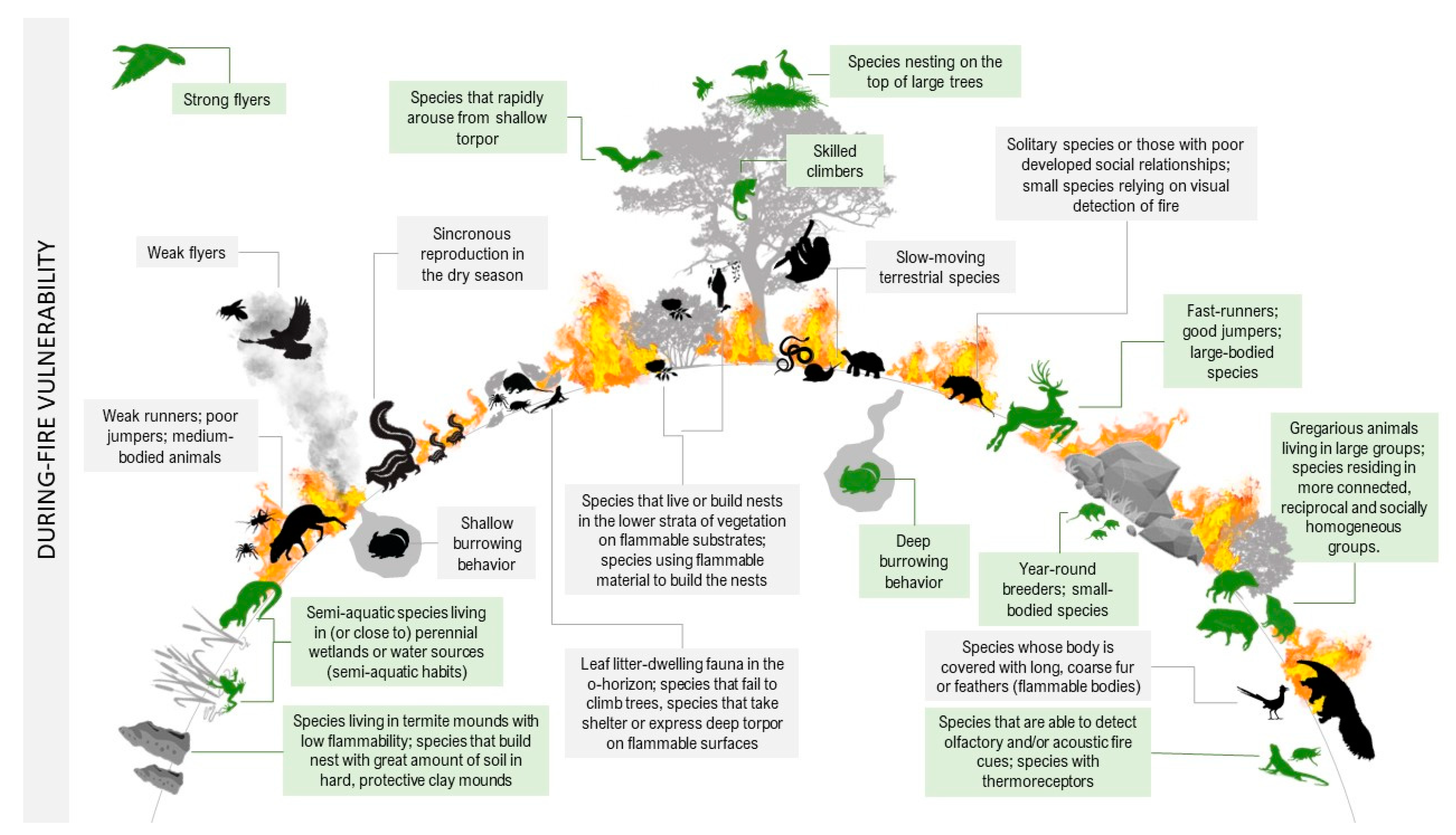
Figure 1. Fire vulnerability traits of species during wildfires. In green are animals that are most likely to survive the burning (decreased sensitivity). In black are animals whose traits increase their probability of death (increased sensitivity).
Dormancy
Being in a torpor during a wildfire may increase or decrease the chances of survival, depending on shelter security and depth of torpor. Low body temperatures are associated with decreased responsiveness (both sensory and locomotor function remains limited) and torpid animals might therefore face an increased mortality risk during fires due to inhalation of toxic smoke, oxygen depletion, and heat exposure [12]. Even though torpid animals can respond to fire stimuli, they may be slow in doing so; therefore, when in deep torpor animals are at risk of not responding to fire cues quickly enough to survive [13].
Increased sensitivity: Species that often present deep torpor on flammable surfaces in the flame zone [14].
Decreased sensitivity: Non-hibernators; species that rapidly arouse from shallow torpor when exposed to fire cues [13][15]; species that remain in torpor in places protected from fire, such as in deeper soil layers.
Escape Decision
When an animal becomes aware of approaching fire, it has two possible escape options: it can move away or find shelter. In the first approach, complex physiological adjustments, which include increases in oxygen consumption, body temperature, heart rate, and blood flow to skeletal muscle prepare the animal for prolonged strenuous activity [11]. As a result, the animal tends to move away from the threat. The alternative behavioral response involves stopping moving, bradycardia, and depression of metabolism [16]. As a result, the animal tends to hide in nearby refuges. Each decision will have different implications for the individual’s survival.
Increased sensitivity: Species that run randomly when frightened can become disoriented when surrounded by fire [10]. In this case, animal survival depends on speed, agility, spatial memory, and a good navigation capacity, which can be compromised when in panic (or under severe stress). However, not only extreme heat but also smoke can affect the animal’s ability to navigate, causing disorientation while trying to escape [17][18][19]. Fossorial species with shallow burrowing behavior may also die, as fire can induce advective flows in soils (e.g., shallow-nesting mining bees) [20][21][22][23]. Species that take shelter in flammable or suffocating places, such as plants in the lower layers, litter, or cavities in small trees [23].
Decreased sensitivity: Species that run toward nearby refuges when frightened. In this case, animal survival depends on how protected refuges are. Small animals using deep burrows or termite mounds as main shelters (e.g., lizards that flee to termite mounds and soil burrows) [24]. Scansorial species that seek refuge on top of tall trees during surface fires, in water, or on rocky surfaces with little flammable material.
Habitat Use
Typically, savanna fires produce flames of 1–2 m in height, which consume all the herbaceous and most of the woody vegetation of about this height [25][26]. Depending on how the species uses the habitat for nesting, foraging, or shelter, it may be more or less exposed to fire. More vulnerable and less mobile stages (e.g., eggs, offspring, larvae, pupae, and pre-emergent adults found in nests) are particularly susceptible to burning. Nest residents may die from lethal substrate heating unless the nests are adequately insulated.
Increased sensitivity: Leaf litter-dwelling fauna in the O-horizon [27][28] and other species that live or build nests in the lower strata of vegetation on flammable substrates, such as shrubs, grasses, dry and/or fallen trunks and branches, and small trees. For example, macroinvertebrate detritivores such as millipedes (Diplopoda), woodlice (Isopoda), and fly larvae (Diptera: Nematocera); fungivorous such as fungus gnats (Diptera: Sciaridae), and predators such as spiders (Araneae), centipedes (Chilopoda), and ground beetles (Coleoptera: Carabidae) [28]; leaf-litter herpetofauna [29][30]. Above-ground nesters that use pre-existing cavities made by other organisms in the thinner branches and smaller trees [31]; species nesting in soil deposits in cracks and crevices of soil-limited landscapes that occur in rock outcrops [32].
Decreased sensitivity: Soil-dwelling species, such as earthworms and worm lizards, that are able to burrow deeper into the ground (10–20 cm deep) [33][34]; species that excavate, use pre-existing holes or natural cavities underground to take shelter, or build nests (below-ground nesters) [35]; species that live or build nests close to perennial wetlands or water sources (aquatic or semi-aquatic habits), on rocky substrates, termite mounds with low flammability, and deep cavities inside massive tree trunks or in the upper strata of vegetation (on the top of tall trees) [36][37][38][39][40].
Mobility
This trait is associated with the species’ ability to flee from the flame zone. Species that exhibit more powerful and flexible movement capabilities should be better able to escape fires.
Increased sensitivity: Nonvolant species of relatively low vagility, including amphibians, snakes, small lizards with short limbs (slow-running animals), and slow-moving animals such as sloths, turtles, and molluscs in general [34][41][42][43][44]; larger arboreal animals that are expected to attach less well to surfaces and have more difficulty distributing loads uniformly across large contact areas [45]; ground-dwelling species that fail to climb trees [7][46]; smaller jumpers with reduced effective jump height [47][48]; weak flyers with shorter wings and smaller flight muscles that usually can only fly a short distance at lower strata of vegetation on the flame zone [49][50].
Decreased sensitivity: Fast runners that can reach higher maximum speeds and escape the flames or travel greater distances, increasing the chances of finding safe shelter away from the fire [51]; birds that can fly higher and avoid the rising column of gasses, smoke, ash, particulates, and other debris produced by a fire [52][53]; lizards with longer limbs, larger toe pads, and more lamellae can run faster, exert stronger cling forces, and perch higher [54]; skilled climbers able to reach the tops of taller trees (at least 4–5 m above) [7][9]; larger jumpers with great effective jump height and other jumping specialists (e.g., arthropods that use catapult mechanisms [47][48].
Morphology
Body size can influence the animal’s ability to find shelter or flee during a fire.
Increased sensitivity: Medium-bodied species may have difficulty fleeing or finding refuge and are more susceptible to direct mortality during the fire or increased chances of predation following a fire [55]. Terrestrial mammals whose bodies are covered with long, coarse fur may be more affected by fire due to the greater flammability of their bodies [56].
Decreased sensitivity: Small-bodied species can find and move into safe micro-refugia (e.g., frogs) [57]; large-bodied species are able to flee or move readily away from the affected areas to avoid direct mortality [55].
Nest Substrate
Beyond location, the substrate used to build nests can increase or decrease the chances of fire spreading.
Increased sensitivity: Species that build nests with thatched mounds [58]; moss and lichen (dry out rapidly because they lack developed root systems) [32][59]; fine grass or mammalian hair (capable of trapping a great deal of air) [60]; plant material such as bark, vegetal fiber, leaves, twigs, grasses, tussocks, and branches [61][62].
Decreased sensitivity: Species that use a great amount of soil in the nests [63][64] to build hard, protective clay mounds (e.g., some termites, wasps, ants, and birds) [65]; species with a deliberate behavior for modifying their surrounding environment, thus reducing flammability (e.g., birds that reduce litter around their nests—‘fuel management’) [66][67]; species that build subterranean nests without thatched mounds [58].
Reproductive Cycles
Pregnant females may experience reduced speed, maneuverability, and endurance [68][69]. This likely occurs due to the additional physical load of the eggs or embryos, which makes the body broader and heavier [70][71][72] and decreases muscle strength [73]. Because they are slower and heavier, pregnant females tend not to endure long runs [74], which increases the likelihood of dying from fire injuries before finding shelter. In addition, pregnant and lactating females tend to spend most of their time stationary and sleeping, avoiding energetically costly behaviors such as running or climbing [75][76][77]. The longer vulnerable life stages are exposed to fire, the greater the risk of individual mortality and population decline.
Increased sensitivity: Species with synchronous reproduction at the end of the dry season, exposing pregnant, lactating, nesting, and brooding females to high-intensity fire (e.g., holometabolous insects whose larvae are restricted to dry-season) [78]. In species with a higher allocation of parental care, females may be burned in an attempt to protect the offspring or by delaying their decision to flee the fire [79]. K-strategists would be particularly disadvantaged because, in addition to longer pregnancies, parental care is more pronounced and offspring tend to depend on their parents for longer [80].
Decreased sensitivity: Species in which the majority of individuals are able to reproduce at any month of the year (year-round breeders); species that reproduce during the wet season but stop reproducing during the dry season. R-strategists would benefit because, in addition to a shorter pregnancy, they generally produce more offspring, which tend to grow at a faster rate to fully utilize the window of favorable environmental conditions with minimal (or no) parental care [80].
Sensory Detection of Fire Cues
Some animals are able to detect fire cues, either through olfactory (chemo-reception of smoke), visual (smoke plumes and flames), or acoustic (crackling sounds) means. Others may rely on thermoreceptors that can detect infrared radiation [11][81][82][83][84]. The greater the detection distance of fire cues, the greater the chance of an animal escaping and surviving. In general, olfactory cues can reach the farthest, followed by auditory and visual cues, which often signal immediate danger [7]. However, the value of fire cues as an early warning signal likely depends on an animal’s sensory sensitivity, an individual’s perceptual range, the fire behavior, and the environmental context [85][86].
Increased sensitivity: Species that spend most of their time surrounded by dense vegetation and rely primarily on the visual detection of fire may be particularly vulnerable, as the visual cues of fire might not enter an animal’s perceptual range until it is very close [85][87][88]. Based on these terms, small-bodied animals could be even more vulnerable, as they usually have lower visual acuity [89].
Decreased sensitivity: Species that are able to detect olfactory and/or acoustic fire cues may have more time to make good escape decisions because they can detect fire at greater distances regardless of vegetation structure [13][15][81][90][91][92]. Species that are able to detect fire cues at lower thresholds (e.g., lower concentrations and from greater distances). Species that rely on thermoreceptors can detect infrared radiation from fires [82][93][94]. Species that spend most of their time on top of tall trees, in open or low-stature vegetation, and in topographically simple landscapes may have some escape advantage as the rising smoke plumes could enter the animal’s perceptual range from a considerable distance (tens of kilometers), providing ample warning of fire risk [85][95].
Social Organization
Vigilance allows animals to monitor their surroundings and detect threats before it is too late to escape; however, it represents an allocation of time and energy that could be devoted to foraging and other fitness-enhancing activities [96]. In that case, sociality seems to be a good strategy because group members can reduce their investment in vigilance at no significant increased risk to themselves. As group size increases, individual vigilance decreases, and yet overall group vigilance and detection ability increases (effect of many eyes scanning for threats) [97]. However, for collective detection to work, it is important that at least one individual is vigilant and detects a threat (in this case, fire), upon which it alerts other group members, whether seeking shelter, fleeing, or emitting alarm calls. The fear responses may be socially transmitted by a cascade effect or contagious alertness [98][99][100]. Therefore, individuals who have not detected the threat by themselves can use this information and still escape before it is too late [101][102]. Therefore, aspects of social organization, such as group size, relationship structure, and communication system can determine the effectiveness of collective detection of fire signals.
Increased sensitivity: Solitary species or those that live in small family groups (parents and young) rely on fewer individuals to detect approaching fire [103]. Species with poorly developed social relationships (e.g., groups with weak connections) and whose individuals or groups lack effective communication skills.
Decreased sensitivity: Gregarious species living in large groups [104][105][106]. Social species residing in more connected, reciprocal, and socially homogeneous groups [107][108][109][110].
2.2. Post Fire
Animals may be indirectly negatively affected after fire by the simplified habitat structure with greater microclimate extremes (e.g., temperature, humidity, and greater exposure to predators), diminished food resources (e.g., less food, lowered nutritional status, or decreased palatability), or interactions with other organisms (e.g., increased competition, predation, or parasitism) [111]. The physical and behavioral traits that can increase or decrease a species’ sensitivity to the indirect effects of fire are described in detail below and illustrated in Figure 2.
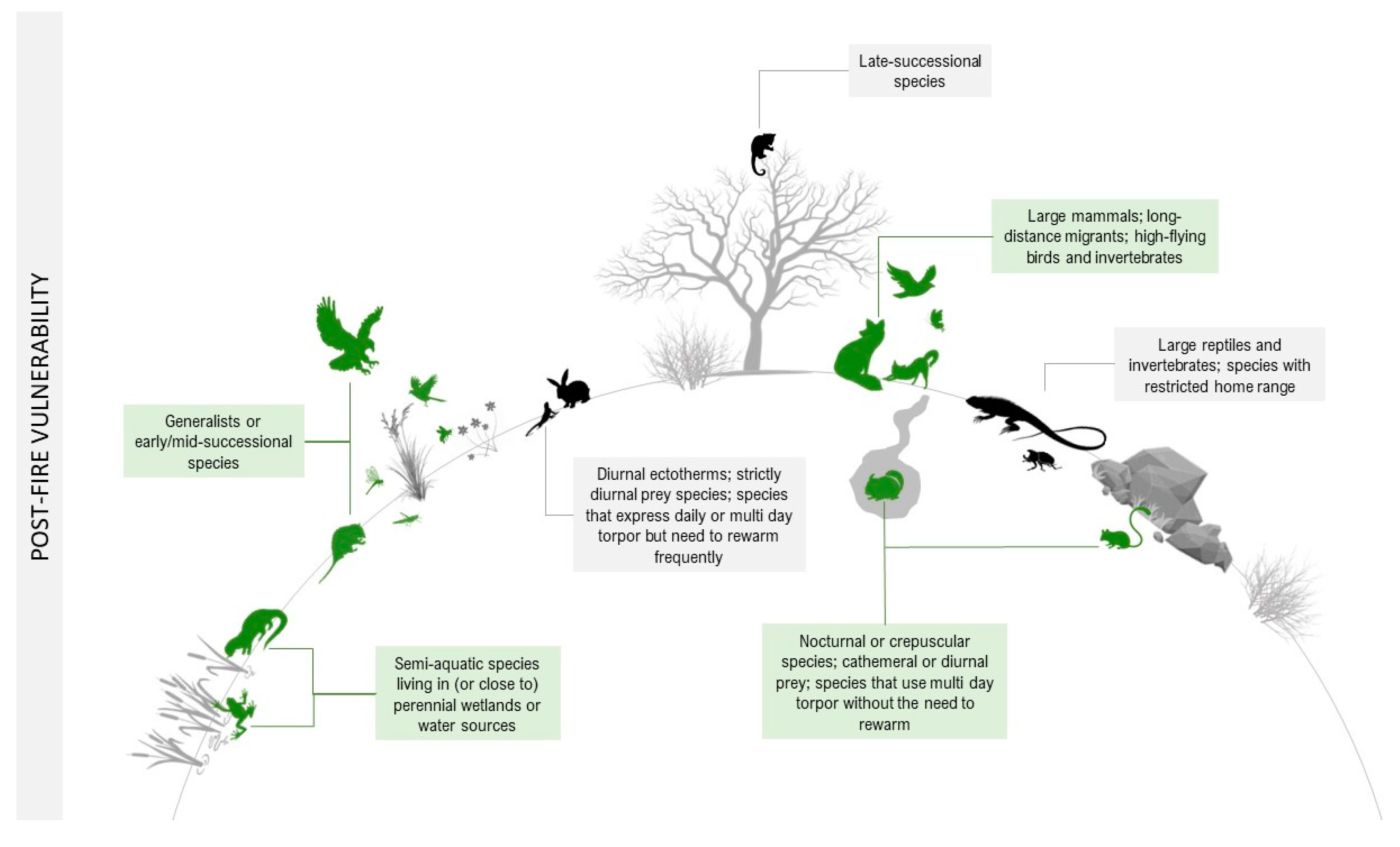
Figure 2. Fire vulnerability traits of species after a fire. In green are the animals most likely to survive in the post-fire landscape (decreased sensitivity). In black are the animals whose traits increase the probability of death after a fire (increased sensitivity).
Behavioral Plasticity
Population recovery depends on the species’ behavioral plasticity with respect to habitat structure and diet.
Increased sensitivity: Late-successional species that require more structured habitats for nest sites and/or foraging, which take several years to recover [112]: canopy and upper-middle strata insectivores that forage in thicker bark or denser canopies [113][114]; small arboreal animals that depend on late successional resources (e.g., leaf litter and thick branches); tree cavity-nesters that rely on highly-decayed wood or large living trees that provide both long-lasting cavities (e.g., in the main stem) and a series of single-use cavities (e.g., in dead branches) [114][115][116]; pollinators, nectarivores, and frugivores that benefit, respectively, from specific late-successional flowers, fruits and seeds of trees and shrubs [113][117][118][119][120]; low mesic insectivores that forage in thick litter [113]; saproxylic insects typically associated with large old trees and the decaying wood they generate [121]; invertebrates that have biological stages of their development inside fungal fruiting bodies [122].
Decreased sensitivity: Generalists that can temporarily adapt their diet and/or habitat preferences to the conditions and food resources available across the post-fire landscape [113]; species that may benefit from fire-induced changes such as predators (birds of prey) [123] and early or mid-successional species: open grassland species [124], aerial insectivores that benefit from the increased availability of flying insects [116][125][126]; nectarivores, frugivores, and granivores that forage on (or close to) the ground and benefit from the greater abundance of small herbaceous plants producing flowers, fruits, and seeds after fire [127][128][129][130][131]; deadwood-associated species [93].
Dormancy
Dormancy allows species to cope with the scorched post-fire environment, avoiding risky foraging movements within the simplified post-fire landscape and reducing the chances of starving or being captured by a predator [132][133][134].
Increased sensitivity: Species that express multi-day torpor but need to rewarm frequently. These species may deplete energy reserves and starve before their preferred habitat and resources recover since active rewarming from torpor requires a substantial increase in energy expenditure and can compromise energy savings gained from using torpor. On the other hand, passive rewarming from torpor involves basking in the sun and, consequently, being more exposed to predators [91][135]. Species that use daily torpor, which lasts only some hours rather than days or weeks, and is usually, but not always, interrupted by daily foraging and feeding. In this case, individuals will have to deal with the lack of food resources, which may impair the ability to rewarm after daily torpor [136]. Additionally, since torpid animals move slower than when normothermic and during foraging, they may be captured by a predator or exposed to altered environmental conditions [92].
Decreased sensitivity: Invertebrates that express aestivation and remain in an inactive stage that is remarkably resistant to water loss (e.g., mucus cocoon to resist desiccation) or that can afford the loss of water and sustain a dry form without compromising on revival upon rehydration (e.g., all anhydrobiotes) [137]; species that use multi-day torpor for weeks or even months after a fire or during fire season without the need to rewarm [14][133][134][136].
Endogenous Circadian Rhythms
Fire-induced changes may affect diurnal, crepuscular, and nocturnal species differently.
Increased sensitivity: Diurnal ectotherms that depend on thermoregulation opportunities [138][139]; strictly diurnal prey species, which become more vulnerable to increased predation rates [133][140][141][142].
Decreased sensitivity: For nocturnal or crepuscular animals, nighttime environmental temperatures are often lower than preferred temperatures. For this reason, individuals seek out warmer places within (or closer to) their preferred temperature range (e.g., deep bark fissures, hollow branches, warm rocks, trees, or inside retreat sites during the day), which are typically more protected from predators [143]. Cathemeral or diurnal prey that can adjust their daily activity patterns [133].
Mobility
Finding new habitat beyond the fire perimeter is likely to be a major determinant of population persistence because if individuals do not disperse they risk reduced fitness or increased mortality due to predation or starvation [133].
Increased sensitivity: Species with restricted home range (e.g., burrows or rock crevices); territorial species with high site fidelity that may perceive the risk of leaving their territory or home range to locate unburned patches to be greater than that of remaining in a familiar area with little or no food resource [18][144][145]. Migratory species (highly mobile) but with strong site fidelity to a limited number of stopover locations and travel routes can have adverse demographic results if traditional sites are completely scorched [146].
Decreased sensitivity: Highly mobile species that travel long distances (e.g., migratory or high-flying birds) or show metapopulation dynamics [84]; nomadic or non-territorial species with low site fidelity [144][145].
Morphology
Increased sensitivity: Large ectotherms. The body size–environment interaction is profound in ectotherms because they rely on external heat [147][148]. Since heat is dissipated more slowly in large-bodied animals (lower surface-to-volume ratio), being large in a post-fire environment may be particularly disadvantageous for an ectotherm as it can be more sensitive to overheating. Invertebrates with thinner cuticles are expected to desiccate faster [137].
Decreased sensitivity: Large mammals [149][150]; species with black morphs or that can change their color after a fire, likely diminishing predator detectability while foraging after a fire [151]; invertebrates with higher cuticle thickness, which gives the animal the advantage of reducing water loss (desiccation resistance) [152].
This entry is adapted from the peer-reviewed paper 10.3390/fire6060242
References
- Beerling, D.J.; Osborne, C.P. The origin of the savanna biome. Glob. Chang. Biol. 2006, 12, 2023–2031.
- Simon, M.F.; Grether, R.; de Queiroz, L.P.; Skema, C.; Pennington, R.T.; Hughes, C.E. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proc. Natl. Acad. Sci. USA 2009, 106, 20359–20364.
- Balch, J.K.; Bradley, B.A.; Abatzoglou, J.T.; Nagy, R.C.; Fusco, E.J.; Mahood, A.L. Human-started wildfires expand the fire niche across the United States. Proc. Natl. Acad. Sci. USA 2017, 114, 2946–2951.
- Le Page, Y.; Morton, D.; Hartin, C.; Bond-Lamberty, B.; Pereira, J.M.C.; Hurtt, G.; Asrar, G. Synergy between land use and climate change increases future fire risk in Amazon forests. Earth Syst. Dyn. 2017, 8, 1237–1246.
- Silva Junior, C.H.L.; Aragão, L.E.O.C.; Fonseca, M.G.; Almeida, C.T.; Vedovato, L.B.; Anderson, L.O. Deforestation-Induced Fragmentation Increases Forest Fire Occurrence in Central Brazilian Amazonia. Forests 2018, 9, 305.
- Pyne, S.J. From Pleistocene to Pyrocene: Fire Replaces Ice. Earth’s Futur. 2020, 8, e2020EF001722.
- Nimmo, D.G.; Carthey, A.J.R.; Jolly, C.J.; Blumstein, D.T. Welcome to the Pyrocene: Animal survival in the age of megafire. Glob. Chang. Biol. 2021, 27, 5684–5693.
- Engstrom, R.T. First-Order Fire Effects on Animals: Review and Recommendations. Fire Ecol. 2010, 6, 115–130.
- Zylstra, P. Quantifying the direct fire threat to a critically endangered arboreal marsupial using biophysical, mechanistic modelling. Austral Ecol. 2022, 48, 266–288.
- Sanderfoot, O.V.; Bassing, S.B.; Brusa, J.L.; Emmet, R.L.; Gillman, S.J.; Swift, K.; Gardner, B. A review of the effects of wildfire smoke on the health and behavior of wildlife. Environ. Res. Lett. 2021, 16, 123003.
- Gutiérrez, J.; De Miguel, J. Fires in nature: A review of the challenges for wild animals. Eur. J. Ecol. 2021, 7, 95–117.
- Recher, H.F.; Lunney, D.; Matthews, A. Small mammal populations in a eucalypt forest affected by fire and drought. I. Long-term patterns in an era of climate change. Wildl. Res. 2009, 36, 143–158.
- Doty, A.C.; Currie, S.E.; Stawski, C.; Geiser, F. Can bats sense smoke during deep torpor? Physiol. Behav. 2018, 185, 31–38.
- Geiser, F.; Stawski, C.; Doty, A.C.; E Cooper, C.; Nowack, J. A burning question: What are the risks and benefits of mammalian torpor during and after fires? Conserv. Physiol. 2018, 6, coy057.
- Mendyk, R.W.; Weisse, A.; Fullerton, W. A wake-up call for sleepy lizards: The olfactory-driven response of Tiliqua rugosa (Reptilia: Squamata: Sauria) to smoke and its implications for fire avoidance behavior. J. Ethol. 2019, 38, 161–166.
- Moberg, G.P.; Mench, J.A. The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; CABI Publishing: New York, NY, USA, 2000.
- Garcês, A.; Pires, I. The Hell of Wildfires: The Impact on Wildlife and Its Conservation and the Role of the Veterinarian. Conservation 2023, 3, 96–108.
- Fagan, W.F.; Lewis, M.A.; Auger-Méthé, M.; Avgar, T.; Benhamou, S.; Breed, G.; LaDage, L.; Schlägel, U.E.; Tang, W.-W.; Papastamatiou, Y.P.; et al. Spatial memory and animal movement. Ecol. Lett. 2013, 16, 1316–1329.
- Branco, T.; Redgrave, P. The Neural Basis of Escape Behavior in Vertebrates. Annu. Rev. Neurosci. 2020, 43, 417–439.
- Massman, W.J.; Frank, J.M.; Mooney, S.J. Advancing Investigation and Physical Modeling of First-Order Fire Effects on Soils. Fire Ecol. 2010, 6, 36–54.
- Cane, J.H.; Neff, J.L. Predicted fates of ground-nesting bees in soil heated by wildfire: Thermal tolerances of life stages and a survey of nesting depths. Biol. Conserv. 2011, 144, 2631–2636.
- Love, B.G.; Cane, J.H. Limited direct effects of a massive wildfire on its sagebrush steppe bee community. Ecol. Èntomol. 2016, 41, 317–326.
- Jordaan, P.R.; Steyl, J.C.A.; Hanekom, C.C.; Combrink, X. Fire-associated reptile mortality in Tembe Elephant Park, South Africa. Fire Ecol. 2020, 16, 4–9.
- Costa, B.M.; Pantoja, D.L.; Vianna, M.C.M.; Colli, G.R. Direct and Short-Term Effects of Fire on Lizard Assemblages from a Neotropical Savanna Hotspot. J. Herpetol. 2013, 47, 502–510.
- Miranda, H.S.; Sato, M.N.; Neto, W.N.; Aires, F.S. Fires in the cerrado, the Brazilian savanna. In Tropical Fire Ecology; Springer: Heidelberg, Germany, 2009; pp. 427–450.
- Rodrigues, C.A.; Zirondi, H.L.; Fidelis, A. Fire frequency affects fire behavior in open savannas of the Cerrado. For. Ecol. Manag. 2021, 482, 118850.
- Kauf, Z.; Damsohn, W.; Fangmeier, A. Do relationships between leaf traits and fire behaviour of leaf litter beds persist in time? PLoS ONE 2018, 13, e0209780.
- Buckingham, S.; Murphy, N.; Gibb, H. Effects of fire severity on the composition and functional traits of litter-dwelling macroinvertebrates in a temperate forest. For. Ecol. Manag. 2018, 434, 279–288.
- Rocha, C.F.D.; Van Sluys, M.; Alves, M.A.S.; Bergallo, H.; Vrcibradic, D. Activity of Leaf-Litter Frogs: When Should Frogs Be Sampled? J. Herpetol. 2000, 34, 285.
- Dundas, S.J.; Ruthrof, K.X.; Hardy, G.E.S.; Fleming, P.A. Some like it hot: Drought-induced forest die-off influences reptile assemblages. Acta Oecologica 2021, 111, 103714.
- Rosa, T.F.; Camarota, F.; Zuanon, L.A.; Tito, R.; Maravalhas, J.B.; Powell, S.; Vasconcelos, H.L. The effects of high-severity fires on the arboreal ant community of a Neotropical savanna. Oecologia 2021, 196, 951–961.
- Markle, C.E.; Wilkinson, S.L.; Waddington, J.M. Initial Effects of Wildfire on Freshwater Turtle Nesting Habitat. J. Wildl. Manag. 2020, 84, 1373–1383.
- Gomes, J.O.; Maciel, A.O.; Costa, J.C.L.; Andrade, G.V. Diet Composition in Two Sympatric Amphisbaenian Species (Amphisbaena ibijara and Leposternon polystegum) from the Brazilian Cerrado. J. Herpetol. 2009, 43, 377–384.
- Certini, G.; Moya, D.; Lucas-Borja, M.E.; Mastrolonardo, G. The impact of fire on soil-dwelling biota: A review. For. Ecol. Manag. 2021, 488, 118989.
- Avitabile, S.C.; Nimmo, D.G.; Bennett, A.F.; Clarke, M.F. Termites Are Resistant to the Effects of Fire at Multiple Spatial Scales. PLoS ONE 2015, 10, e0140114.
- Robinson, N.M.; Leonard, S.W.; Ritchie, E.; Bassett, M.; Chia, E.K.; Buckingham, S.; Gibb, H.; Bennett, A.F.; Clarke, M.F. Review: Refuges for fauna in fire-prone landscapes: Their ecological function and importance. J. Appl. Ecol. 2013, 50, 1321–1329.
- Simioni, F.; Campos, V.A.; Dorado-Rodrigues, T.F.; Penha, J.; Strüssmann, C. Crab burrows and termite thermal chimneys as refuges for anurans in a neotropical wetland. Salamandra 2014, 50, 133–138.
- Monteiro, I.; Viana-Junior, A.B.; Solar, R.R.D.C.; Neves, F.D.S.; DeSouza, O. Disturbance-modulated symbioses in termitophily. Ecol. Evol. 2017, 7, 10829–10838.
- Schmidt, I.B.; Fidelis, A.; Miranda, H.S.; Ticktin, T. How do the wets burn? Fire behavior and intensity in wet grasslands in the Brazilian savanna. Braz. J. Bot. 2016, 40, 167–175.
- Costa, L.M.; de Freitas, G.H.S.; da Silva, P.H.V.B.P.; Ribeiro, L.C.; de Vasconcelos, M.F.; Rodrigues, M. Breeding biology of the Cipo Cinclodes Cinclodes espinhacensis, a micro-endemic furnariid of the southeastern Brazilian mountains. Rev. Bras. Ornitol. 2019, 27, 63–69.
- Tomas, W.M.; Berlinck, C.N.; Chiaravalloti, R.M.; Faggioni, G.P.; Strüssmann, C.; Libonati, R.; Abrahão, C.R.; Alvarenga, G.D.V.; Bacellar, A.E.D.F.; Batista, F.R.D.Q.; et al. Distance sampling surveys reveal 17 million vertebrates directly killed by the 2020′s wildfires in the Pantanal, Brazil. Sci. Rep. 2021, 11, 23547.
- Trochet, A.; Le Chevalier, H.; Calvez, O.; Barthe, L.; Isselin-Nondedeu, F.; Picard, D.; Debelgarric, M.; Pégourié, N.; Rocher, R.; Ribéron, A. Postbreeding Movements in Marbled Newts (Caudata, Salamandridae): A Comparative Radiotracking Study in Two Habitat Types. Herpetologica 2017, 73, 1–9.
- Enriquez-Urzelai, U.; Bernardo, N.; Moreno-Rueda, G.; Montori, A.; Llorente, G. Are amphibians tracking their climatic niches in response to climate warming? A test with Iberian amphibians. Clim. Chang. 2019, 154, 289–301.
- Brand, M.E.; Rechkemmer, W.T.; Clark, S.A.; McCravy, K.W.; Lydeard, C.; Meiers, S.T.; Jenkins, S.E. The Influence of Fire and Other Environmental Factors on Terrestrial Gastropod Species Composition in an Oak-Hickory Woodland of West-Central Illinois. Am. Malacol. Bull. 2020, 38, 39–49.
- Labonte, D.; Federle, W. Scaling and biomechanics of surface attachment in climbing animals. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140027.
- Jolly, C.J.; Dickman, C.R.; Doherty, T.S.; van Eeden, L.M.; Geary, W.L.; Legge, S.M.; Woinarski, J.C.Z.; Nimmo, D.G. Animal mortality during fire. Glob. Chang. Biol. 2022, 28, 2053–2065.
- Sutton, G.P.; Mendoza, E.; Azizi, E.; Longo, S.J.; Olberding, J.P.; Ilton, M.; Patek, S.N. Why do Large Animals Never Actuate Their Jumps with Latch-Mediated Springs? Because They can Jump Higher without Them. Integr. Comp. Biol. 2019, 59, 1609–1618.
- Brandt, E.E.; Sasiharan, Y.; Elias, D.O.; Mhatre, N. Jump takeoff in a small jumping spider. J. Comp. Physiol. A 2021, 207, 153–164.
- Sarremejane, R.; Mykrä, H.; Bonada, N.; Aroviita, J.; Muotka, T. Habitat connectivity and dispersal ability drive the assembly mechanisms of macroinvertebrate communities in river networks. Freshw. Biol. 2017, 62, 1073–1082.
- Mardiastuti, A. Response and impact of fire on bird community in the tropical rainforest: A review. IOP Conf. Ser. Earth Environ. Sci. 2020, 504, 012001.
- Hirt, M.R.; Jetz, W.; Rall, B.C.; Brose, U. A general scaling law reveals why the largest animals are not the fastest. Nat. Ecol. Evol. 2017, 1, 1116–1122.
- Weyand, P.G.; Davis, J.A. Running performance has a structural basis. J. Exp. Biol. 2005, 208, 2625–2631.
- Ruf, T.; Valencak, T.; Tataruch, F.; Arnold, W. Running Speed in Mammals Increases with Muscle n-6 Polyunsaturated Fatty Acid Content. PLoS ONE 2006, 1, e65.
- Winchell, K.M.; Maayan, I.; Fredette, J.R.; Revell, L.J. Linking locomotor performance to morphological shifts in urban lizards. Proc. R. Soc. B Boil. Sci. 2018, 285, 20180229.
- Griffiths, A.D.; Brook, B.W. Effect of fire on small mammals: A systematic review. Int. J. Wildland Fire 2014, 23, 1034–1043.
- Silveira, L.; Henrique, F.; Rodrigues, G.; Jácomo, A.T.d.A.; Filho, J.A.F.D. Impact of wildfires on the megafauna of Emas National Park, central Brazil. Oryx 1999, 33, 108–114.
- Mahony, M.; Gould, J.; Beranek, C.T.; Callen, A.; Clulow, J.; Clulow, S.; Klop-Toker, K.; Mahony, S.; Wallace, S.; Seeto, R.; et al. A trait-based analysis for predicting impact of wildfires on frogs. Aust. Zool. 2022, 42, 326–351.
- Jofré, L.E.; Curth, M.D.T.; Farji-Brener, A.G. Unexpected costs of extended phenotypes: Nest features determine the effect of fires on leaf cutter ant’s demography. Proc. R. Soc. B Boil. Sci. 2022, 289, 20212333.
- Moore, P.A.; Smolarz, A.G.; Markle, C.E.; Waddington, J.M. Hydrological and thermal properties of moss and lichen species on rock barrens: Implications for turtle nesting habitat. Ecohydrology 2018, 12, e2057.
- Collias, N. Engineering aspects of nest building by birds. Endeavour 1986, 10, 9–16.
- Costa, L.M.; de Freitas, G.H.S.; Rodrigues, M. Architecture, composition and placement of nests of the Cipo Canastero Asthenes luizae (Aves: Furnariidae), a bird endemic to Brazilian mountaintops. J. Nat. Hist. 2019, 53, 391–412.
- Alambiaga, I.; Álvarez, E.; Diez-Méndez, D.; Verdejo, J.; Barba, E. “The tale of the three little tits”: Different nest building solutions under the same environmental pressures. Avian Biol. Res. 2020, 13, 49–56.
- Rowley, I. The use of mud in nest-building—A review of the incidence and taxonomic importance. Ostrich 1969, 40, 139–148.
- Abensperg-Traun, M.; Milewski, A.V. Abundance and diversity of termites (Isoptera) in imburnt versus burnt vegetation at the Barrens in Mediterranean Western Australia. Aust. J. Ecol. 1995, 20, 413–417.
- Wijas, B.J.; Lim, S.; Cornwell, W.K. Continental-scale shifts in termite diversity and nesting and feeding strategies. Ecography 2021, 2022, e05902.
- Smith, A.; Avitabile, S.C.; Leonard, S.W.J. Less fuel for the fire: Malleefowl (Leipoa ocellata) nesting activity affects fuel loads and fire behaviour. Wildl. Res. 2016, 43, 640.
- Maisey, A.C.; Haslem, A.; Leonard, S.W.J.; Bennett, A.F. Foraging by an avian ecosystem engineer extensively modifies the litter and soil layer in forest ecosystems. Ecol. Appl. 2020, 31, e02219.
- Cooper, W.E., Jr.; Vitt, L.J.; Hedges, R.; Huey, R. Locomotor impairment and defense in gravid lizards (Eumeces laticeps): Behavioral shift in activity may offset costs of reproduction in an active forager. Behav. Ecol. Sociobiol. 1990, 27, 153–157.
- Smith, S.K.; Young, V.K.H. Balancing on a Limb: Effects of Gravidity on Locomotion in Arboreal, Limbed Vertebrates. Integr. Comp. Biol. 2021, 61, 573–578.
- Veasey, J.S.; Houston, D.C.; Metcalfe, N.B. A hidden cost of reproduction: The trade-off between clutch size and escape take-off speed in female zebra finches. J. Anim. Ecol. 2001, 70, 20–24.
- Guillemette, M.; Ouellet, J.-F. Temporary flightlessness as a potential cost of reproduction in pre-laying Common Eiders Somateria mollissima. IBIS 2005, 147, 301–306.
- Fokidis, H.B.; Risch, T.S. The burden of motherhood: Gliding locomotion in mammals influences maternal reproductive investment. J. Mammal. 2008, 89, 617–625.
- Noren, S.R.; Redfern, J.V.; Edwards, E.F. Pregnancy is a drag: Hydrodynamics, kinematics and performance in pre- and post-parturition bottlenose dolphins (Tursiops truncatus). J. Exp. Biol. 2011, 214, 4151–4159.
- Miles, D.B.; Sinervo, B.; Frankino, W.A. Reproductive burden, locomotor performance, and the cost of reproduction in free ranging lizards. Evolution 2000, 54, 1386–1395.
- Voigt, C.C. Reproductive energetics of the nectar-feeding bat Glossophaga soricina (Phyllostomidae). J. Comp. Physiol. B 2003, 173, 79–85.
- Miller, K.E.; Bales, K.L.; Ramos, J.H.; Dietz, J.M. Energy intake, energy expenditure, and reproductive costs of female wild golden lion tamarins (Leontopithecus rosalia). Am. J. Primatol. 2006, 68, 1037–1053.
- Speakman, J.R. The physiological costs of reproduction in small mammals. Philos. Trans. R. Soc. B Biol. Sci. 2007, 363, 375–398.
- Nascimento, A.R.; Malinov, I.K.; Freire, G.; Freitas, A.V.L.; Diniz, I.R. The Temporal Dynamics of Two Morpho Fabricius, 1807 Species (Lepidoptera: Nymphalidae) are Affected Differently by Fire in the Brazilian Savanna. Environ. Èntomol. 2020, 49, 1449–1454.
- Blumstein, D.T.; Hayes, L.D.; Pinter-Wollman, N. Social consequences of rapid environmental change. Trends Ecol. Evol. 2022, 38, 337–345.
- Daly, M.; Wilson, M. Sex, Evolution and Behavior, 2nd ed; Willard Grant Press: Boston, MA, USA, 1983.
- Grafe, T.U.; Döbler, S.; Linsenmair, K.E. Frogs flee from the sound of fire. Proc. R. Soc. B Boil. Sci. 2002, 269, 999–1003.
- Schmitz, H.; Bousack, H. Modelling a Historic Oil-Tank Fire Allows an Estimation of the Sensitivity of the Infrared Receptors in Pyrophilous Melanophila Beetles. PLoS ONE 2012, 7, e37627.
- Álvarez-Ruiz, L.; Belliure, J.; Pausas, J.G. Fire-driven behavioral response to smoke in a Mediterranean lizard. Behav. Ecol. 2021, 32, 662–667.
- Álvarez-Ruiz, L.; Pausas, J.G.; Blumstein, D.T.; Putman, B.J. Lizards’ response to the sound of fire is modified by fire history. Anim. Behav. 2023, 196, 91–102.
- Nimmo, D.G.; Avitabile, S.; Banks, S.C.; Bird, R.B.; Callister, K.; Clarke, M.F.; Dickman, C.R.; Doherty, T.S.; Driscoll, D.A.; Greenville, A.C.; et al. Animal movements in fire-prone landscapes. Biol. Rev. 2018, 94, 981–998.
- Pausas, J.G.; Parr, C.L. Towards an understanding of the evolutionary role of fire in animals. Evol. Ecol. 2018, 32, 113–125.
- Prevedello, J.A.; Forero-Medina, G.; Vieira, M.V. Does land use affect perceptual range? Evidence from two marsupials of the Atlantic Forest. J. Zool. 2011, 284, 53–59.
- Prevedello, J.A.; Forero-Medina, G.; Vieira, M.V. Movement behaviour within and beyond perceptual ranges in three small mammals: Effects of matrix type and body mass. J. Anim. Ecol. 2010, 79, 1315–1323.
- Kiltie, R.A. Scaling of visual acuity with body size in mammals and birds. Funct. Ecol. 2000, 14, 226–234.
- Álvarez, G.; Ammagarahalli, B.; Hall, D.R.; Pajares, J.A.; Gemeno, C. Smoke, pheromone and kairomone olfactory receptor neurons in males and females of the pine sawyer Monochamus galloprovincialis (Olivier) (Coleoptera: Cerambycidae). J. Insect Physiol. 2015, 82, 46–55.
- Stawski, C.; Matthews, J.K.; Körtner, G.; Geiser, F. Physiological and behavioural responses of a small heterothermic mammal to fire stimuli. Physiol. Behav. 2015, 151, 617–622.
- Nowack, J.; Delesalle, M.; Stawski, C.; Geiser, F. Can hibernators sense and evade fires? Olfactory acuity and locomotor performance during deep torpor. Sci. Nat. 2016, 103, 73.
- Schütz, S.; Weissbecker, B.; Hummel, H.E.; Apel, K.-H.; Schmitz, H.; Bleckmann, H. Insect antenna as a smoke detector. Nature 1999, 398, 298–299.
- Schmitz, H.; Schmitz, A.; Kreiss, E.; Gebhardt, M.; Gronenberg, W. Navigation to Forest Fires by Smoke and Infrared Reception: The Specialized Sensory Systems of “Fire-Loving” Beetles. Navigation 2008, 55, 137–145.
- Kay, G.M.; Driscoll, D.A.; Lindenmayer, D.B.; Pulsford, S.A.; Mortelliti, A. Pasture height and crop direction influence reptile movement in an agricultural matrix. Agric. Ecosyst. Environ. 2016, 235, 164–171.
- Beauchamp, G. On how risk and group size interact to influence vigilance. Biol. Rev. 2019, 94, 1918–1934.
- van der Marel, A.; López-Darias, M.; Waterman, J.M. Group-enhanced predator detection and quality of vigilance in a social ground squirrel. Anim. Behav. 2019, 151, 43–52.
- Decety, J.; Norman, G.J.; Berntson, G.G.; Cacioppo, J.T. A neurobehavioral evolutionary perspective on the mechanisms underlying empathy. Prog. Neurobiol. 2012, 98, 38–48.
- Morelli, F.; Benedetti, Y.; Díaz, M.; Grim, T.; Ibáñez-Álamo, J.D.; Jokimäki, J.; Kaisanlahti-Jokimäki, M.; Tätte, K.; Markó, G.; Jiang, Y.; et al. Contagious fear: Escape behavior increases with flock size in European gregarious birds. Ecol. Evol. 2019, 9, 6096–6104.
- Socias-Martínez, L.; Kappeler, P.M. Catalyzing Transitions to Sociality: Ecology Builds on Parental Care. Front. Ecol. Evol. 2019, 7, 160.
- Beauchamp, G.; Li, Z.; Yu, C.; A Bednekoff, P.; Blumstein, D.T. A meta-analysis of the group-size effect on vigilance in mammals. Behav. Ecol. 2021, 32, 919–925.
- Pérez-Manrique, A.; Gomila, A. Emotional contagion in nonhuman animals: A review. WIREs Cogn. Sci. 2021, 13, e1560.
- Clutton-Brock, T.H.; Gaynor, D.; McIlrath, G.M.; Maccoll, A.D.C.; Kansky, R.; Chadwick, P.; Manser, M.; Skinner, J.D.; Brotherton, P.N.M. Predation, group size and mortality in a cooperative mongoose, Suricata suricatta. J. Anim. Ecol. 1999, 68, 672–683.
- Komarek, E.V. Fire and animal behavior. In Proceedings of the Tall Timbers Fire Ecology Conference, Tallahassee, FL, USA, 10–11 April 1969; pp. 160–207.
- Pruetz, J.D.; LaDuke, T.C. Brief communication: Reaction to fire by savanna chimpanzees (Pan troglodytes verus) at Fongoli, Senegal: Conceptualization of “fire behavior” and the case for a chimpanzee model. Am. J. Phys. Anthr. 2009, 141, 646–650.
- Arganda, S.; Pérez-Escudero, A.; de Polavieja, G.G. A common rule for decision making in animal collectives across species. Proc. Natl. Acad. Sci. USA 2012, 109, 20508–20513.
- DeSouza, O.; Albuquerque, L.B.; Tonello, V.M.; Pinto, L.P.; Junior, R.R. Effects of fire on termite generic richness in a savanna-like ecosystem (‘Cerrado’) of Central Brazil. Sociobiology 2003, 42, 639–649.
- Ellis, S.; Snyder-Mackler, N.; Ruiz-Lambides, A.; Platt, M.L.; Brent, L.J.N. Deconstructing sociality: The types of social connections that predict longevity in a group-living primate. Proc. R. Soc. B Boil. Sci. 2019, 286, 20191991.
- Montero, A.P.; Williams, D.M.; Martin, J.G.; Blumstein, D.T. More social female yellow-bellied marmots, Marmota flaviventer, have enhanced summer survival. Anim. Behav. 2020, 160, 113–119.
- Philson, C.S.; Blumstein, D.T. Emergent social structure is typically not associated with survival in a facultatively social mammal. Biol. Lett. 2023, 19, 20220511.
- Pilon, N.A.L.; Cava, M.G.B.; Hoffmann, W.A.; Abreu, R.C.R.; Fidelis, A.; Durigan, G. The diversity of post-fire regeneration strategies in the cerrado ground layer. J. Ecol. 2020, 109, 154–166.
- van Mantgem, E.F.; Keeley, J.E.; Witter, M. Faunal Responses to Fire in Chaparral and Sage Scrub in California, USA. Fire Ecol. 2015, 11, 128–148.
- Gosper, C.R.; Prober, S.M.; Yates, C.J. Estimating fire interval bounds using vital attributes: Implications of uncertainty and among-population variability. Ecol. Appl. 2013, 23, 924–935.
- Latif, Q.S.; Saab, V.A.; Dudley, J.G. Prescribed fire limits wildfire severity without altering ecological importance for birds. Fire Ecol. 2021, 17, 37.
- Haslem, A.; Avitabile, S.C.; Taylor, R.S.; Kelly, L.T.; Watson, S.; Nimmo, D.G.; Kenny, S.A.; Callister, K.; Spence-Bailey, L.M.; Bennett, A.F.; et al. Time-since-fire and inter-fire interval influence hollow availability for fauna in a fire-prone system. Biol. Conserv. 2012, 152, 212–221.
- Rush, S.; Klaus, N.; Keyes, T.; Petrick, J.; Cooper, R. Fire severity has mixed benefits to breeding bird species in the southern Appalachians. For. Ecol. Manag. 2012, 263, 94–100.
- Chalmandrier, L.; Midgley, G.; Barnard, P.; Sirami, C. Effects of time since fire on birds in a plant diversity hotspot. Acta Oecologica 2013, 49, 99–106.
- Lee, J.S.; Cornwell, W.K.; Kingsford, R.T. Rainforest bird communities threatened by extreme fire. Glob. Ecol. Conserv. 2021, 33, e01985.
- Rainsford, F.W.; Kelly, L.T.; Leonard, S.W.; Bennett, A.F. Post-fire habitat relationships for birds differ among ecosystems. Biol. Conserv. 2021, 260, 109218.
- Robinson, N.M.; Leonard, S.W.; Bennett, A.F.; Clarke, M.F. Refuges for birds in fire-prone landscapes: The influence of fire severity and fire history on the distribution of forest birds. For. Ecol. Manag. 2014, 318, 110–121.
- Graf, M.; Lettenmaier, L.; Müller, J.; Hagge, J. Saproxylic beetles trace deadwood and differentiate between deadwood niches before their arrival on potential hosts. Insect Conserv. Divers. 2021, 15, 48–60.
- Paviour-Smith, K. The Fruiting-Bodies of Macrofungi as Habitats for Beetles of the Family Ciidae (Coleoptera). Oikos 1960, 11, 43.
- Hovick, T.J.; McGranahan, D.A.; Elmore, R.D.; Weir, J.R.; Fuhlendorf, S.D. Pyric-carnivory: Raptor use of prescribed fires. Ecol. Evol. 2017, 7, 9144–9150.
- Lopes, L.E.; de Meireles, R.C.; Peixoto, H.J.C.; Teixeira, J.P.G.; Machado, T.L.d.S.S.; Lombardi, V.T. Movement ecology of the threatened Campo Miner Geositta poeciloptera and its implications for the conservation of tropical open grassland birds. Bird Conserv. Int. 2022, 33, E38.
- Bagne, K.E.; Purcell, K.L. Short-term responses of birds to prescribed fire in fire-suppressed forests of California. J. Wildl. Manag. 2011, 75, 1051–1060.
- Walesiak, M.; Mikusiński, G.; Borowski, Z.; Żmihorski, M. Large fire initially reduces bird diversity in Poland’s largest wetland biodiversity hotspot. Biodivers. Conserv. 2022, 31, 1037–1056.
- McGee, J.M. Small Mammal Populations in an Unburned and Early Fire Successional Sagebrush Community. J. Range Manag. 1982, 35, 177.
- Briani, D.C.; Palma, A.R.; Vieira, E.M.; Henriques, R.P. Post-fire succession of small mammals in the Cerrado of central Brazil. Biodivers. Conserv. 2004, 13, 1023–1037.
- Holmes, A.L.; Robinson, W.D. Fire mediated patterns of population densities in mountain big sagebrush bird communities. J. Wildl. Manag. 2013, 77, 737–748.
- Beal-Neves, M.; Chiarani, E.; Ferreira, P.M.A.; Fontana, C.S. The role of fire disturbance on habitat structure and bird communities in South Brazilian Highland Grasslands. Sci. Rep. 2020, 10, 19708.
- Saab, V.A.; Latif, Q.R.; Block, W.M.; Dudley, J.G. Short-term benefits of prescribed fire to bird communities of dry forests. Fire Ecol. 2022, 18, 4.
- Stawski, C.; Hume, T.; Körtner, G.; Currie, S.E.; Nowack, J.; Geiser, F. Post-fire recovery of torpor and activity patterns of a small mammal. Biol. Lett. 2017, 13, 20170036.
- Matthews, J.K.; Stawski, C.; Körtner, G.; Parker, C.A.; Geiser, F. Torpor and basking after a severe wildfire: Mammalian survival strategies in a scorched landscape. J. Comp. Physiol. B 2016, 187, 385–393.
- Stawski, C.; Körtner, G.; Nowack, J.; Geiser, F. The importance of mammalian torpor for survival in a post-fire landscape. Biol. Lett. 2015, 11, 20150134.
- Geiser, F.; Drury, R.L.; Körtner, G.; Turbill, C.; Pavey, C.R.; Brigham, R.M. Passive rewarming from torpor in mammals and birds: Energetic, ecological and evolutionary implications. In Life in the Cold: Evolution, Mechanisms, Adaptation, and Application; Barnes, B.M., Carey, H.V., Eds.; Biological Papers of the University of Alaska: Fairbanks, AK, USA, 2004; pp. 51–62.
- Turbill, C.; Stojanovski, L. Torpor reduces predation risk by compensating for the energetic cost of antipredator foraging behaviours. Proc. R. Soc. B Boil. Sci. 2018, 285, 20182370.
- Thorat, L.; Nath, B.B. Insects with Survival Kits for Desiccation Tolerance under Extreme Water Deficits. Front. Physiol. 2018, 9, 1843.
- Legge, S.; Murphy, S.; Heathcote, J.; Flaxman, E.; Augusteyn, J.; Crossman, M. The short-term effects of an extensive and high-intensity fire on vertebrates in the tropical savannas of the central Kimberley, northern Australia. Wildl. Res. 2008, 35, 33–43.
- Lindsay, M.N.; Lewis, D.B.; Halstead, N.; Gainsbury, A.M. Fire severity effects on the herpetofaunal diversity of the Florida scrub, a biodiversity hotspot. Biodivers. Conserv. 2023, 32, 1857–1878.
- Leahy, L.; Legge, S.; Tuft, K.; McGregor, H.; Barmuta, L.; Jones, M.; Johnson, C.N. Amplified predation after fire suppresses rodent populations in Australia’s tropical savannas. Wildl. Res. 2015, 42, 705–716.
- Shaw, R.E.; James, A.I.; Tuft, K.; Legge, S.; Cary, G.J.; Peakall, R.; Banks, S.C. Unburnt habitat patches are critical for survival and in situ population recovery in a small mammal after fire. J. Appl. Ecol. 2021, 58, 1325–1335.
- Doherty, T.S.; Geary, W.L.; Jolly, C.J.; Macdonald, K.J.; Miritis, V.; Watchorn, D.J.; Cherry, M.J.; Conner, L.M.; González, T.M.; Legge, S.M.; et al. Fire as a driver and mediator of predator–prey interactions. Biol. Rev. 2022, 97, 1539–1558.
- Nordberg, E.J.; Schwarzkopf, L. Reduced competition may allow generalist species to benefit from habitat homogenization. J. Appl. Ecol. 2018, 56, 305–318.
- Switzer, P.V. Site fidelity in predictable and unpredictable habitats. Evol. Ecol. 1993, 7, 533–555.
- Kreling, S.E.; Gaynor, K.M.; McInturff, A.; Calhoun, K.L.; Brashares, J.S. Site fidelity and behavioral plasticity regulate an ungulate’s response to extreme disturbance. Ecol. Evol. 2021, 11, 15683–15694.
- Klaassen, M.; Bauer, S.; Madsen, J.; Tombre, I. Modelling behavioural and fitness consequences of disturbance for geese along their spring flyway. J. Appl. Ecol. 2005, 43, 92–100.
- Sagonas, K.; Meiri, S.; Valakos, E.D.; Pafilis, P. The effect of body size on the thermoregulation of lizards on hot, dry Mediterranean islands. J. Therm. Biol. 2013, 38, 92–97.
- Zamora-Camacho, F.J.; Reguera, S.; Moreno-Rueda, G. Bergmann’s Rule rules body size in an ectotherm: Heat conservation in a lizard along a 2200-metre elevational gradient. J. Evol. Biol. 2014, 27, 2820–2828.
- Prada, M.; Marinho-Filho, J. Effects of Fire on the Abundance of Xenarthrans in Mato Grosso, Brazil. Austral Ecol. 2004, 29, 568–573.
- Turschak, G.; Rochester, C.; Hathaway, S.; Stokes, D.; Haas, C.; Fisher, R. Effects of Large-Scale Wildfire on Carnivores in San Diego County, California. San Diego, CA. 2010. Available online: https://sdmmp.com/view_article.php?cid=CiteID_1603251358357600 (accessed on 15 March 2021).
- Miranda, R.B.; Klaczko, J.; Tonini, J.F.; Brandão, R.A. Escaping from predators: A review of Neotropical lizards defence traits. Ethol. Ecol. Evol. 2022, 34, 1–31.
- Céréghino, R.; Françoise, L.; Bonhomme, C.; Carrias, J.-F.; Compin, A.; Corbara, B.; Jassey, V.; Leflaive, J.; Rota, T.; Farjalla, V.; et al. Desiccation resistance traits predict freshwater invertebrate survival and community response to drought scenarios in a Neotropical ecosystem. Ecol. Indic. 2020, 119, 106839.
This entry is offline, you can click here to edit this entry!
