Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Developmental Biology
Dopamine (DA) and dopamine agonists (DA-Ag) are known for their therapeutic effects in diseases involving neurochemical alterations in the nervous system. However, these compounds have different biochemical properties that allow them to be applied to treat other diseases, as is the case with their antiangiogenic effect, a property that can be applied to treat pathologies where angiogenesis is an important physiological mechanism, such as cancer, endometriosis, and osteoarthritis (OA).
- antiangiogenic
- cancer
- dopamine (DA)
- dopamine agonists (DA-Ag)
- endometriosis
- osteoarthritis (OA)
- vascular endothelial growth factor (VEGF)
- vascular endothelial growth factor receptor (VEGFR)
1. Antiangiogenic Capacity of Dopamine (DA) and Dopamine Agonists (DA-Ag) and the Mechanisms of Action
Angiogenesis, the generation of new blood vessels from the existing vasculature, plays a key role in physiological processes such as embryonic development, wound healing, and organ regeneration, as well as in various pathologies, such as cancer, diabetes, retinopathies, and tumor metastasis [15,66]. Several molecular mechanisms have been explored to understand the basic processes underlying angiogenesis. Signaling mediated by VEGF and its target receptors has been identified as an important player in angiogenesis and vascular permeability, among others [66,67]. The VEGF family genes are composed of five members, including VEGF-A (VEGF), VEGF-B, VEGF-D, and placental growth factor [68,69,70,71], whereas VEGFR is represented by three members, namely, VEGFR 1, VEGFR 2, and VEGFR 3, where VEGFR 2 is the main regulator of physiological and pathological angiogenesis [15,72,73]. The signal transduction cascade begins when VEGF binds to VEGFR 2, leading to a conformational change, dimerization, and phosphorylation of tyrosine residues of the receptor, which leads to the activation of several intracellular molecules that serve as downstream signaling elements that propagate the signal to activate angiogenesis [72]. This system mediates angiogenesis through the proliferation, migration, and survival of endothelial cells, promoting new vessel formation (Figure 1) [74,75]. The potent proangiogenic activity of VEGF was first described as essential for vascular endothelial cells; however, VEGF and VEGF receptors are expressed on numerous nonendothelial cells, including tumor cells [72,76]. In addition, VEGFR 2 is associated with the mitogenic, angiogenic, and permeability-enhancing effects of VEGF in a wide variety of tissues [75,77].
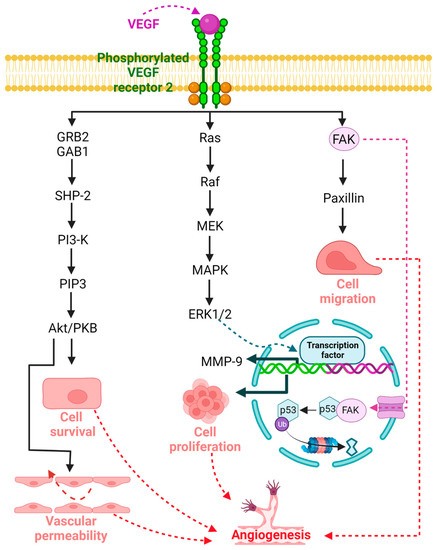
Figure 1. Signaling mediated by vascular endothelial growth factor (VEGF) and its target receptor VEGFR 2 plays an important role in angiogenesis under both physiological and pathological conditions. The signal transduction cascade begins when VEGF binds to VEGFR 2, leading to a conformational change, dimerization, and phosphorylation of tyrosine residues of the receptor, resulting in the activation of several intracellular molecules that act as downstream signaling elements involved in cell survival, vascular permeability, proliferation, and cell migration that promote angiogenesis.
DA and DA-Ag (e.g., bromocriptine, cabergoline, quinagolide, and quinpirole) have demonstrated antiangiogenic properties in different pathologies [14,78,79,80,81,82]. Sarkar et al. demonstrated that DA administered intraperitoneally (50 mg/kg/day) in mice with colon cancer was able to inhibit angiogenesis and tumor growth without apparent adverse effects [83]. This antiangiogenic capacity has been associated with the VEGF pathway [66,67,83], and several mechanisms have been described. For example, Basu et al. reported that DA and its D2R DA-Ag, bromocriptine, and quinpirole inhibited mouse ovarian tumor-induced angiogenesis and inhibited human umbilical vein endothelial cell proliferation and migration [14]. The authors described, for the first time, the antiangiogenic relationship of the DA-D2R/VEGF-VEGFR 2 mechanism, with the induction of VEGFR 2 endocytosis being the key act for the arrest of angiogenesis by DA [14]. Indeed, the internalization and inactivation of VEGFR 2 downregulate several proangiogenic factors and upregulate antiangiogenic factors, resulting in an unstructured blood supply (Figure 2) [80].
Another D2R-related antiangiogenic mechanism was observed in tumor and normal endothelial cells. Normal endothelial cells show very low or no expression of DA-D2R compared to tumor endothelial cells [14,84]. Through paracrine signaling, VEGF secreted by tumor cells can stimulate D2R expression by activating the extracellular-signal-regulated kinase1/2 (ERK1/2) signaling cascade and increasing Krüppel-like factor 11 (KLF11) expression in endothelial cells (Figure 2) [79]. Increased D2R can inhibit VEGF-induced angiogenesis [14,84,85] as a feedback mechanism that regulates the actions of VEGF on endothelial cells [79].
Furthermore, through its D2R, DA not only has an effect on decreasing angiogenesis [86,87], but it also inhibits tumor endothelial cell proliferation through the inactivation of VEGF-induced mitogen-activated protein kinase (MAPK) and focal adhesion kinase (FAK) phosphorylation (Figure 2) [78,88]. FAK is a tyrosine kinase that promotes p53 degradation via ubiquitination, leading to tumor cell growth and proliferation, angiogenesis, and vascular permeability [89]. In addition, D2R receptors can decrease matrix metalloprotease (MMP-9) (ERK1/2-mediated) release by endothelial progenitor cells, inhibiting their mobilization from the bone marrow and preventing their participation in tumor neovascularization [88,90,91].
Moreover, it has been reported that DA can inhibit VEGF-induced endothelial cell migration. By acting through D2R, DA can regulate the phosphorylation of different tyrosine residues of VEGFR 2, leading to the inactivation of different downstream signaling pathways [15,92,93]. Sinha et al., 2009, in isolated human umbilical cord endothelial cells, demonstrated that treatment with 10 μM DA prior to VEGF stimulation at 10 ng/mL produced an increase in the VEGF-induced phosphorylation of phosphatase-2 containing Src homology region 2 domain (SHP-2) and its phosphatase activity. Active SHP-2 dephosphorylates VEGFR 2 at Y951, Y996, and Y1059 but not at Y1175 (15). The decreased phosphorylation of VEGFR 2 at Y951 leads to a subsequent decrease in Src phosphorylation at Y418 and its kinase activity, inhibiting cell migration (Figure 2) [15]. SHP-2 knockdown was also observed to affect the DA-regulated inhibition of VEGF-induced VEGFR 2 phosphorylation and, subsequently, the activation of Src, a protein related to cancer progression [15,94].
Figure 2 shows an integrated and simplified scheme of the main antiangiogenic mechanisms of the DA-D2R/VEGF-VEGFR 2 system in different pathologies. These mechanisms can occur in isolation or together in different cells, organs, or diseases. Future research should be carried out to clarify this question.
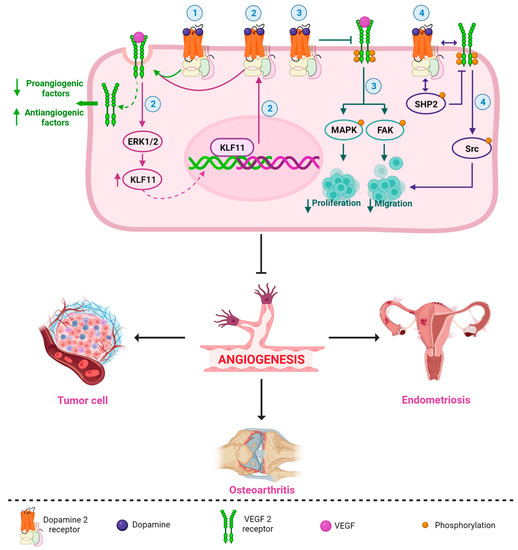
Figure 2. Simplified and integrated scheme of the main antiangiogenic mechanisms described for the DA-D2R/VEGF-VEGFR 2 system in different pathologies. DA (and its DA-Ag, such as bromocriptine, cabergoline, and quinagolide) strongly and selectively inhibit VEGF/VEGFR 2 functions; for example, (1) DA/D2R promote the induction of VEGFR 2 endocytosis, which reduces a series of proangiogenic factors and increases antiangiogenic factors [14,80]; (2) VEGF can stimulate D2R expression by activating the ERK1/2 signaling cascade and upregulating KLF transcription factor 11 expression, and upregulation of D2R can inhibit VEGF-induced angiogenesis (perhaps by promoting VEGFR 2 endocytosis) [79]; (3) DA/D2R inhibits VEGF-induced activation of MAPK and FAK phosphorylation, blocking cell proliferation and migration [88]; and (4) DA may regulate dephosphorylation of different tyrosine residues of VEGFR 2, leading to inactivation of different downstream signaling pathways. DA causes an increased association of D2R with VEGFR 2. DA also induces an increase in the association between SHP-2 (a protein phosphatase) and D2R and stimulates the phosphorylation of SHP-2. Then, active SHP-2 inhibits the phosphorylation of VEGFR 2. The decrease in VEGFR 2 phosphorylation leads to a subsequent decrease in Src phosphorylation, blocking VEGF-induced migration [15]. All these mechanisms (separately or together) may be involved in the inhibition of angiogenesis in different diseases, such as endometriosis, cancer, and osteoarthritis.
2. Therapeutic Potential of Dopamine (DA) and Dopamine Agonists (DA-Ag) as Antiangiogenic Agents
As mentioned above, cancer, endometriosis, and OA are diseases in which angiogenesis is an important physiological mechanism; therefore, the development and maintenance of these conditions can be affected by antiangiogenic therapy, a promising strategy that in the last decade has had an increasing number of studies in which different antiangiogenic agents have been used to inhibit tumor growth, induce the regression of endometriotic lesions, and inhibit osteogenesis by targeting their blood supply [83,84,85,95,96,97]. Drugs related to the VEGF/VEGFR 2 signaling pathway, phytochemicals, immunomodulators, antihormonal drugs, and DA-Ag have been used for their antiangiogenic capacity [3,80,81,98]. Of relevance has been the use of monoclonal antibodies targeting the VEGF pathway for cancer therapy, where studies have shown them to be key inhibitors of tumor angiogenesis during adjuvant, maintenance, or combination therapy against some solid tumors [99,100]. However, not all these compounds have demonstrated safety or tolerability; therefore, it would be important to test antiangiogenic treatments or adjunctive treatments for endometriosis, cancer, or OA with compounds that have been shown to have a favorable safety profile and are already clinically approved for the treatment of other diseases [80,101]. In this context, since DA and DA-Ag have an acceptable safety profile and are clinically approved, they may represent an alternative to many antiangiogenic agents.
2.1. Therapeutic Potential of Dopamine and Dopamine Agonists in Endometriosis
Endometriosis is a common gynecologic disease characterized by the presence of endometrial tissue, glands, and stroma outside the uterine cavity and is a common estrogen-dependent disorder associated with pelvic pain and infertility. Its etiology is unknown, and treatment is surgical with a high risk of recurrence [81,95,102]. Although many aspects of the pathogenesis of endometriosis are not fully established, endometriotic lesions grow in areas with a constant and abundant blood supply, and angiogenesis is a prerequisite for the invasion, proliferation, long-term growth, and maintenance of ectopic implants [81,98,103]. Under this rationale, the use of commercial antiangiogenic drugs has been explored in preclinical models of endometriosis; however, endometriosis specifically affects women of reproductive age, and the selection of antiangiogenic agents is very important, as physiological angiogenic processes such as follicle maturation, corpus luteum function, eutopic endometrial proliferation, and embryo development must be carefully protected [98,101].
DA and DA-Ag have shown a benign clinical profile and several advantages for women with endometriosis, as they are already used for hyperprolactinemia and lactation suppression, do not seem to interfere with physiologic angiogenesis in reproductive organs, and do not interfere with ovulation and spontaneous pregnancy [3,80,96]. The ergot cabergoline and bromocriptine and the nonergot quinagolide are the main D2R DA-Ag tested in different preclinical and clinical studies [82,104,105]. In experimental models of endometriosis, these DA-Ag have been shown to downregulate a series of proangiogenic factors and upregulate antiangiogenic factors in inflammatory, endothelial, and endometrial cells, targeting the newly formed and mature vasculature and resulting in an unstructured blood supply and reduction in lesion size [80].
2.2. Therapeutic Potential of Dopamine and Dopamine Agonists in Cancer
It has been shown that D2R is upregulated in many cancers, and the use of D2R DA-Ag has an anticancer efficacy. The protein and gene expression of D2R was observed in patient samples or cell lines with different types of breast, cervical, brain, and lung cancers. The use of D2R DA-Ag affects different metabolic processes, including autophagy, apoptosis, survival signaling, and proliferation, showing that the use of these drugs as anticancer agents might have chemotherapeutic utility [2]. In relation to the angiogenesis process, it is well established that DA-Ag decreases tumor angiogenesis by inhibiting VEGFR 2-mediated signaling in endothelial cells. Previous studies have shown that DA inhibits the proliferation and migration of the VEGF-induced endothelial cell line HUVEC [117,118,119,120]. The D2R agonist stopped the growth of lung cancer in a human xenograft model, and some of the beneficial antiangiogenic effects of D2R DA-Ag may occur through the inhibition of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (responsible for producing reactive oxygen species) since it promotes angiogenesis [86]. Regarding catecholamine tumor studies, DA, by acting through D2R, inhibits angiogenesis by suppressing the action of the vascular permeability factor and VEGF [121] in both adult endothelial cells and endothelial progenitor cells. In contrast, norepinephrine and epinephrine, by acting through β-adrenoceptors, promote the synthesis of proangiogenic factors in tumor cells [122]. Angiogenesis is related to tumor growth. In aggressive cancer, the blood supply is increased, and endothelial epinephrine cells mobilize from the bone marrow to the tumor site. DA in different kinds of cancer reduces both angiogenic mechanisms [87,123]. Subsequently, further studies with DA-Ag showed the capability to increase D2R expression in endothelial cells, promoting the internalization of VEGFR 2 (Figure 2). Endothelial cells and macrophages reduce VEGF expression and release into peritoneal fluid. In addition, the availability of plasminogen activator inhibitor-1 decreases, which improves fibrinolysis and diminishes angiogenesis [80]. The antiangiogenic activity of cabergoline is expressed in two ways. First, the interaction of cabergoline with D2Rs results in a reduction in prolactin (PLR) cell function, causing a general and local decrease in PRL levels [124]. Cabergoline causes a decrease in PRL, leading to hemoxygenase-1-dependent angiogenesis in macrophages due to decreased VEGF levels. Second, the interaction of cabergoline with D2R leads to the disruption of VEGF binding to its receptor VEGFR 2 and the blockage of VEGF and VEGFR 2 transcription, resulting in an antiangiogenic effect [112]. The antiangiogenic effect of DA and DA-Ag has been demonstrated in vitro and in vivo in different types of cancer; however, more research is still required in this regard.
2.3. Therapeutic Potential of Dopamine and Dopamine Agonists in Osteoarthritis
One of the fundamental characteristics of OA is the wear and tear of articular cartilage. During this process, the reactivation of chondrocyte maturation toward hypertrophy occurs, resulting in bone formation at the edges of the articular surface (osteophytes) [134,135,136]. Articular cartilage maintains a stable phenotype throughout life; however, with aging or articular cartilage injury, OA appears. Healthy articular cartilage lacks nerve endings and blood vessels, which form simultaneously with bone formation during OA [137,138]. Because of the formation of these nerve endings, OA is a chronic pain condition; however, the role of neurotransmitters during OA is just beginning to be understood [139,140,141,142,143,144]. Although cartilage lacks nerve endings, chondrocytes have been found to express some catecholamine receptors, and the role of DA during bone formation has been studied in vitro and also in fracture models in adult individuals [140,145,146]. Another important aspect in the pathogenesis of OA is inflammation, which is exacerbated in the initial phase of OA, mainly by cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNFα), which can also contribute to the damage of articular chondrocytes and ultimately to the dedifferentiation of chondrocytes to a fibrocartilaginous phenotype and finally to bone formation [144,147,148,149].
DA has an anti-inflammatory effect; it has been shown to inhibit the production of proinflammatory cytokines such as interleukin-6 (IL-6), TNFα, and inducible nitric oxide synthase (iNOS) induced by lipopolysaccharide in microglia, and this appears to occur through blocking nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling [150]. This led us to believe that the role of DA in OA would have a “protective” role on articular cartilage damage in vivo and in vitro models of OA. In a study performed in an in vitro experimental model of OA that consisted of treating chondrocytes with IL-1β, it was shown that in DA-treated chondrocytes, they also upregulate the NF-κB and Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3) signaling pathways, inhibiting their nuclear activation and leading to the inhibition of articular cartilage damage markers such as iNOS, cyclooxygenase-2 (COX-2), metalloproteases-1, -3 and -13 (MMP-1, MMP-3, and MMP-13), while markers of healthy articular cartilage such as type II collagen and glycosaminoglycan content were downregulated [139]. In the same work, they found that in an animal model of OA, in mice with destabilization of the medial meniscus, when treated with DA, the effects of joint damage were reversed.
Articular cartilage is an avascular tissue, but in OA, when the synovial membrane becomes inflamed, angiogenesis begins, which is the appearance of blood vessels in the articular cartilage, accelerating the process of bone formation on the cartilage surface with subsequent osteophyte formation [151,152]. Angiogenesis can be caused by synovitis from inflammatory cells such as macrophages that secrete VEGF [153] and in turn induce other cell types such as endothelial cells and fibroblasts to secrete other angiogenic factors such as TNFα and IL-1β [154,155]. On the other hand, angiopoietin plays a regulatory role in angiogenesis by controlling cartilage vascularization [156], and this angiopoietin under normal conditions is produced by synoviocytes. Cartilage degradation accompanies pannus formation and is regulated by the activity of MMP-3, MMP-9, and MMP-13 [157,158,159,160,161], which promote the IL-1β-promoted turnover of the extracellular matrix [149,162,163,164]. Under normal conditions, articular chondrocytes produce antiangiogenic factors such as Troponin-1 and Chondromodulin-1, among others, as well as inhibitors of metalloproteinase [159,165,166,167]. In contrast, hypertrophic chondrocytes present receptors for angiogenic factors, which initiates the last phase of the endochondral ossification in long bones, but in joints, this only occurs when chondrocytes are damaged [166,168]. Another important factor regulating angiogenesis is hypoxia-inducible factor (HIF-1), which joint chondrocytes need to survive in a hostile environment with low oxygen levels (hypoxia) [169,170]; if these levels increase, then the transcription factor SRY-box transcription factor 9 (SOX-9) decreases its expression, and chondrocytes rapidly mature into hypertrophic chondrocytes [171,172], which is an important step in endochondral ossification but also in the establishment of OA. Additionally, the adipokine visfatin has been reported to increase VEGF-dependent angiogenesis, and patients with OA have elevated visfatin levels [173].
The regulation of angiogenesis is thus one of the important points for the treatment of OA. Thus, VEGF signaling may be one of the main therapeutic targets for this disease. The inhibition of VEGF signaling can reduce the progression of OA, and the use of bevacizumab, which is an antibody against VEGF, inhibited angiogenesis and the progression of OA in a rabbit model of OA and increased the thickness and quality of articular cartilage [174,175]. In addition, how miR-485-5p, which is the shRNA of visfatin, inhibits angiogenesis and OA progression in a rat model of OA has been studied [173]. All this creates opportunities for researchers to design treatments that block angiogenesis in OA patients and reduce articular cartilage damage.
It is possible that during OA, DA may act on stem cells that contribute to osteophyte formation during OA, as several papers show how DA inhibits mesenchymal stem cell migration through its D2R [176] and may contribute to bone mass loss and inhibit osteogenesis [97], as it has also been shown to suppress rat bone marrow stem cell differentiation through the protein kinase B/Glycogen synthase kinase-3 beta/β-catenin (AKT/GSK-3β/β-catenin) pathway. Thus, the modulation of DA receptors in osteoblasts has also been proposed as a possible therapy to induce healing in those with rheumatoid arthritis [140] and possibly osteoporosis. We think that, in contrast, the activation or application of DA can regulate the wear and deterioration of articular cartilage in different mechanisms, such as by regulating inflammation, controlling chondrocyte maturation toward hypertrophy, and consequently inhibiting osteoblast formation on the articular surface, where osteophytes are usually formed from fibrochondrocytes or bone marrow stem cells; additionally, we think that DA can reduce angiogenesis through D2R, which suggests that using DA can be an important antiangiogenic strategy to treat OA, but further studies are needed to clarify this issue.
Another indication that DA contributes to cartilage maintenance may be due to a reciprocal role in the sonic hedgehog (Shh) signaling pathway, as it is well known that Shh is required for dopaminergic neuronal development [177,178], rat bone marrow mesenchymal stem cells express dopaminergic genes, and Indian hedgehogs control chondrocyte differentiation and maturation during skeletogenesis. However, the role of the hedgehog pathway is controversial; although there is much evidence that the overactivation of the pathway leads to OA pathogenesis, the complete abrogation of the pathway also results in the same problem [179,180,181,182].
Although there are indications that DA may help reduce articular cartilage wear and reduce pain, few treatments for OA are being applied. One study proposed crosslinked hyaluronic acid infiltration with DA to improve joint lubrication and repair articular cartilage [4]. However, studies have not focused on demonstrating the impact of DA on the inhibition of angiogenesis in OA.
This entry is adapted from the peer-reviewed paper 10.3390/ijms241210199
This entry is offline, you can click here to edit this entry!
