Pheromones are chemical signals secreted by one individual that can affect the behaviors of other individuals within the same species. Ascaroside is an evolutionarily conserved family of nematode pheromones that play an integral role in the development, lifespan, propagation, and stress response of nematodes. Their general structure comprises the dideoxysugar ascarylose and fatty-acid-like side chains. Ascarosides can vary structurally and functionally according to the lengths of their side chains and how they are derivatized with different moieties. Ascarosides (ASCRs) represent the majority of the pheromones secreted by nematodes. The molecular formula for an ascaroside, C33H68O4, was first proposed by Schulz and Becker in 1933. Different phenotypes of nematode species are produced by different ascarosides or combinations of ascarosides; even slight changes in the chemical structure tend to produce drastically different patterns of activity. As a rule, the patterns of the biosynthesis of ascarosides are linked to the phylogeny, lifestyle, and ecological niche of the organism. In addition, different concentrations of the same ascarosides can have different effects on nematodes. Other chemicals such as vanillic acid function as pheromones in some nematodes, but there have been comparatively few studies and discoveries in this area.
- nematode pheromones
- ascarosides
- structures
- functions
1. Development-Related Pheromones Secreted by Nematodes
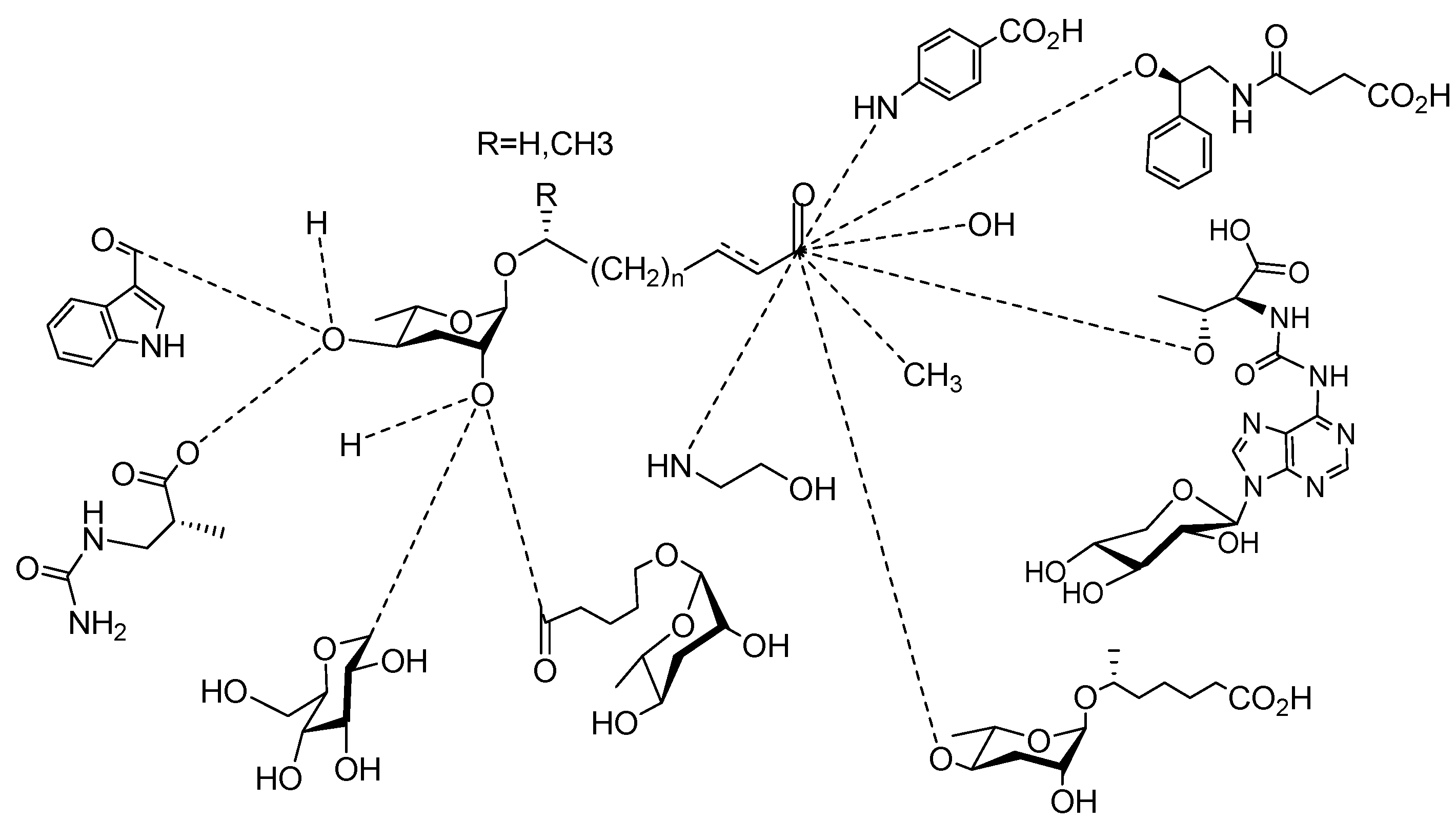
2. Sex Pheromones Secreted by Nematodes
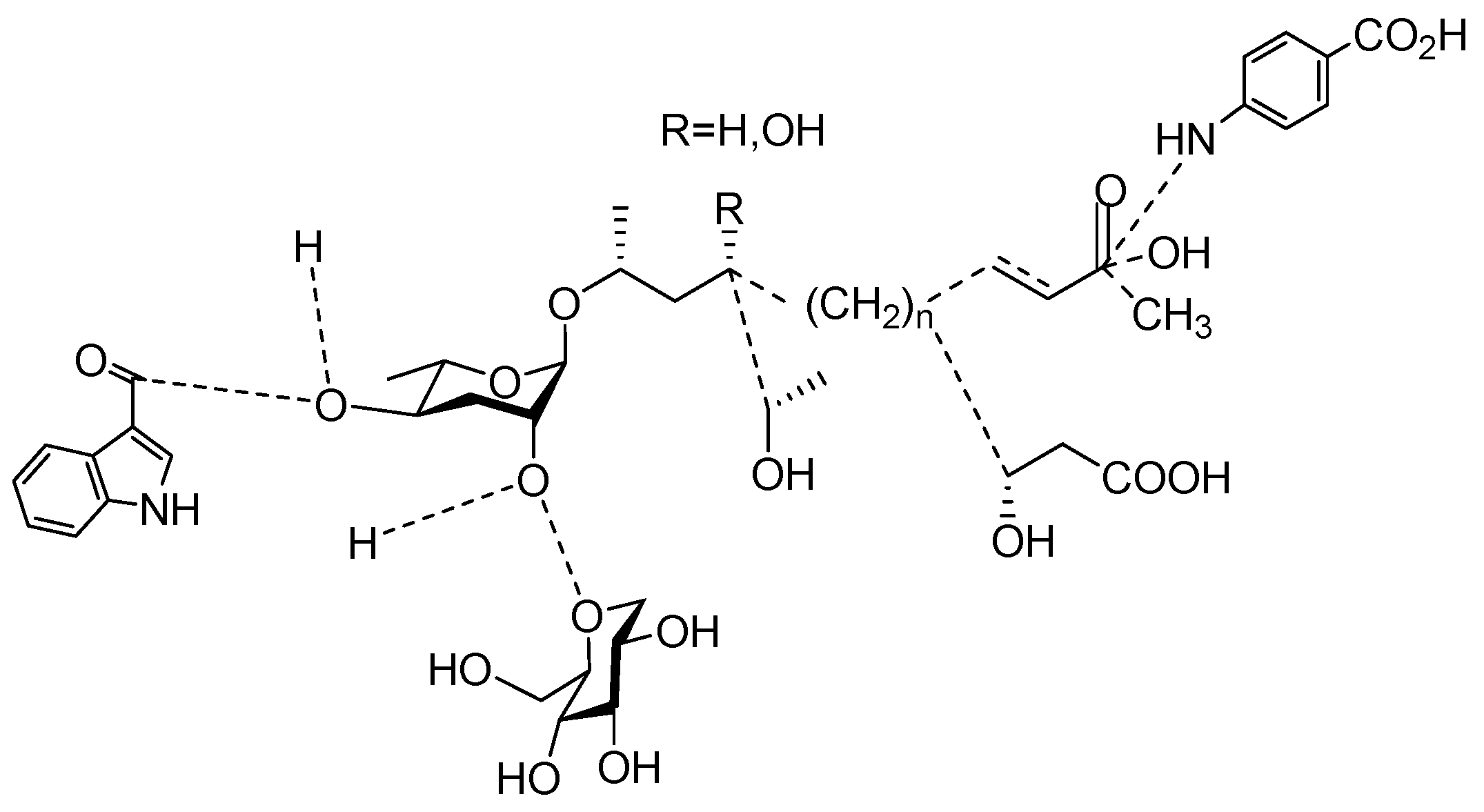
3. Aggregation of Pheromones Secreted by Nematodes
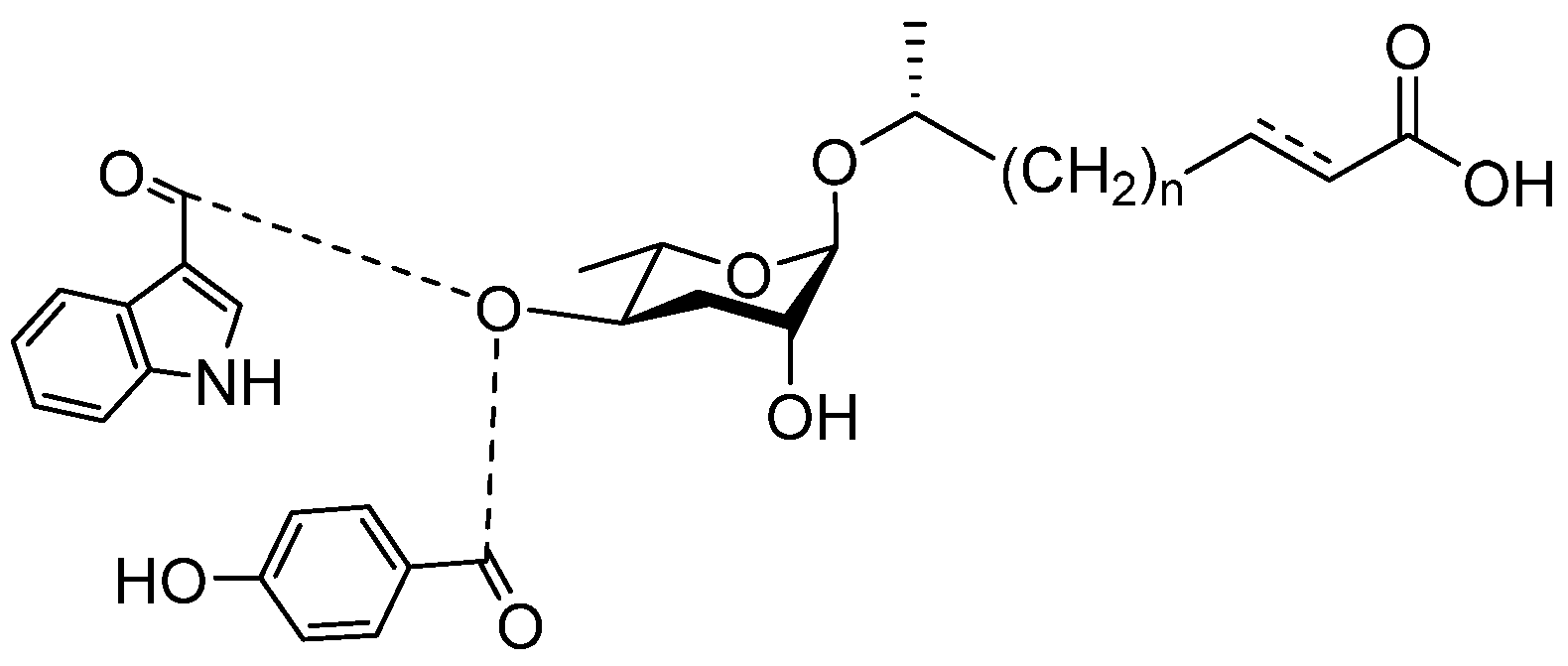
4. Pheromones with Other Functions Secreted by Nematodes
| Name | Chemical Constitution | Function | Organism | Reference | |
|---|---|---|---|---|---|
| Asc C11 EA | 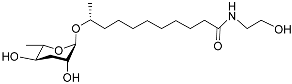 |
Development | C. elegans | [29] | |
| Ascr#1 | 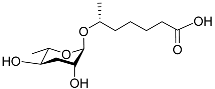 |
Development; mating | C. elegans, P. pacificus, P. redivivus, and Rhabditis sp. SB347 | [9][10][22][24][25][38][47] | |
| Ascr#2 | 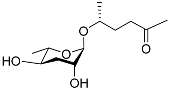 |
Development, mating, foraging, and dispersal | C. elegans; C. briggsae | [10][11][34][35][63] | |
| Ascr#3 | 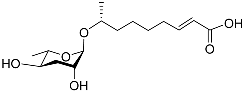 |
Development, mating, foraging, and dispersal | C. elegans | [10][11][12][34][35][36][52][54][55][56][58][59][60][63][64] | |
| Ascr#4 | 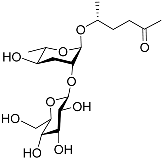 |
Development; mating | C. elegans | [12][34][35] | |
| Ascr#5 | 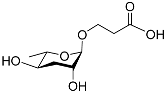 |
Development; foraging | C. elegans | [13][14][15][63] | |
| Ascr#6.1 | 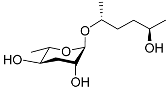 |
Mating | C. elegans | [34] | |
| Ascr#6.2 | 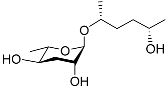 |
Mating | C. elegans | [34] | |
| Ascr#8 | 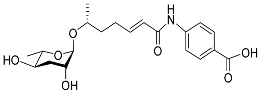 |
Development, mating, foraging, and dispersal | C. elegans | [12][17][34][35][36][63] | |
| Ascr#9 | 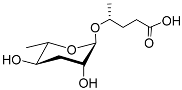 |
Mating; dispersal | C. elegans, P. pacificus, Rhabditis sp. SB347, B. xylophilus, B. mucronatus H. bacteriophora, H.zealandica, H. floridensis, S. carpocapsae, S. riobrave, S. diaprepesi, and S. feltiae |
[22][36][47][65][66][67][68][69] | |
| Ascr#10 | 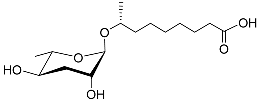 |
Mating | C. elegans; P. redivivus | [37][38][39][40][41][42][43][70] | |
| Ascr#11 | 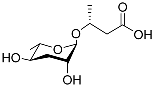 |
Dispersal | C. elegans, S. carpocapsae, S. riobrave, S. diaprepesi, and S. feltiae | [65][66][67][71] | |
| Ascr#12 | 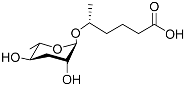 |
Development | C. elegans; P. pacificus | [22][36][69][72] | |
| Ascr#18 | 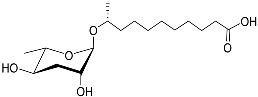 |
Avoidance | M. incognita, M. javanica, M. hapla, H. glycines, and P. brachyurus | [51][70] | |
| Bhas#10 | 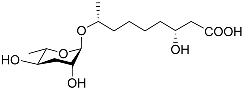 |
Mating | C. elegans; P. redivivus | [38] | |
| Bhas#18 | 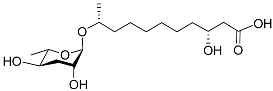 |
Unknown | P. redivivus | [38] | |
| Dasc#1 | 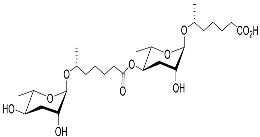 |
Development | P. pacificus | [22][24][25] | |
| Dhas#18 | 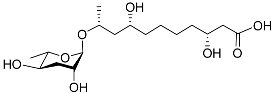 |
Mating | P. redivivus | [38] | |
| Hbas#3 | 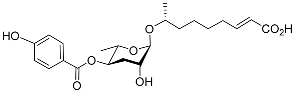 |
Aggregation | C. elegans | [51] | |
| Icas#2 | 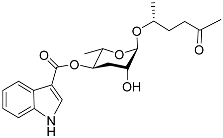 |
Mating | C. briggsae | [44] | |
| Icas#3 | 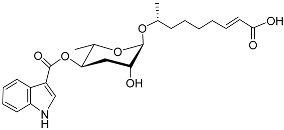 |
Aggregation | C. elegans | [49][50] | |
| Icas#6.2 | 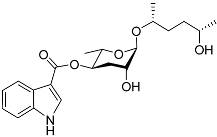 |
Mating | C. briggsae | [44] | |
| Icas#9 | 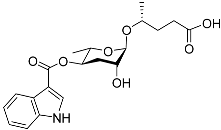 |
Development, aggregation, foraging, and dispersal | C. elegans | [35][49][51][63][73] | |
| Mbas#3 | 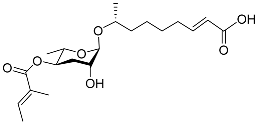 |
Dispersal | C. elegans | [51][58] | |
| Nacq#1 | 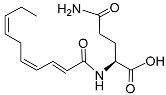 |
Development | C. elegans | [74] | |
| Nacq#2 | 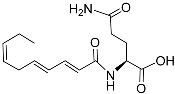 |
Unknown | C. elegans | [74] | |
| Npar#1 | 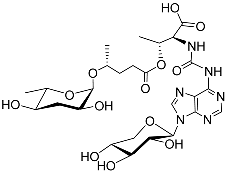 |
Development | P. pacificus | [22][24][25] | |
| Osas#2 | 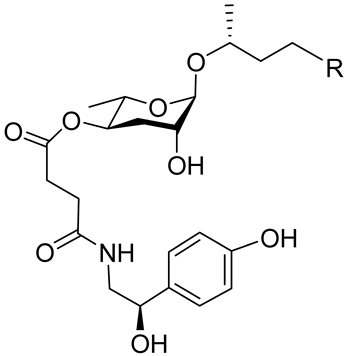 |
R=(C=O)CH3 | Dispersal | C. elegans | [35] |
| Osas#10 | R=(CH2)4COOH | Dispersal | C. elegans | [35] | |
| Osas#9 | 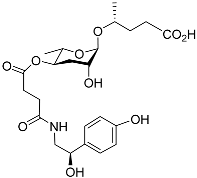 |
Dispersal | C. elegans | [35][53] | |
| Part#9 | 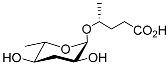 |
Development | P. pacificus | [22][24][25] | |
| Pasc#9 | 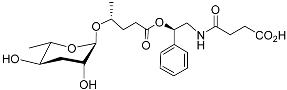 |
Development | P. pacificus | [22][24][25] | |
| Ubas#1 | 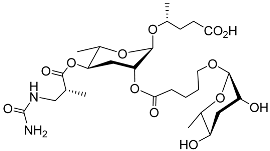 |
Development | P. pacificus | [22][24][25] | |
| Vanillic acid | 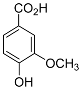 |
Mating | H. glycines | [75][76] | |
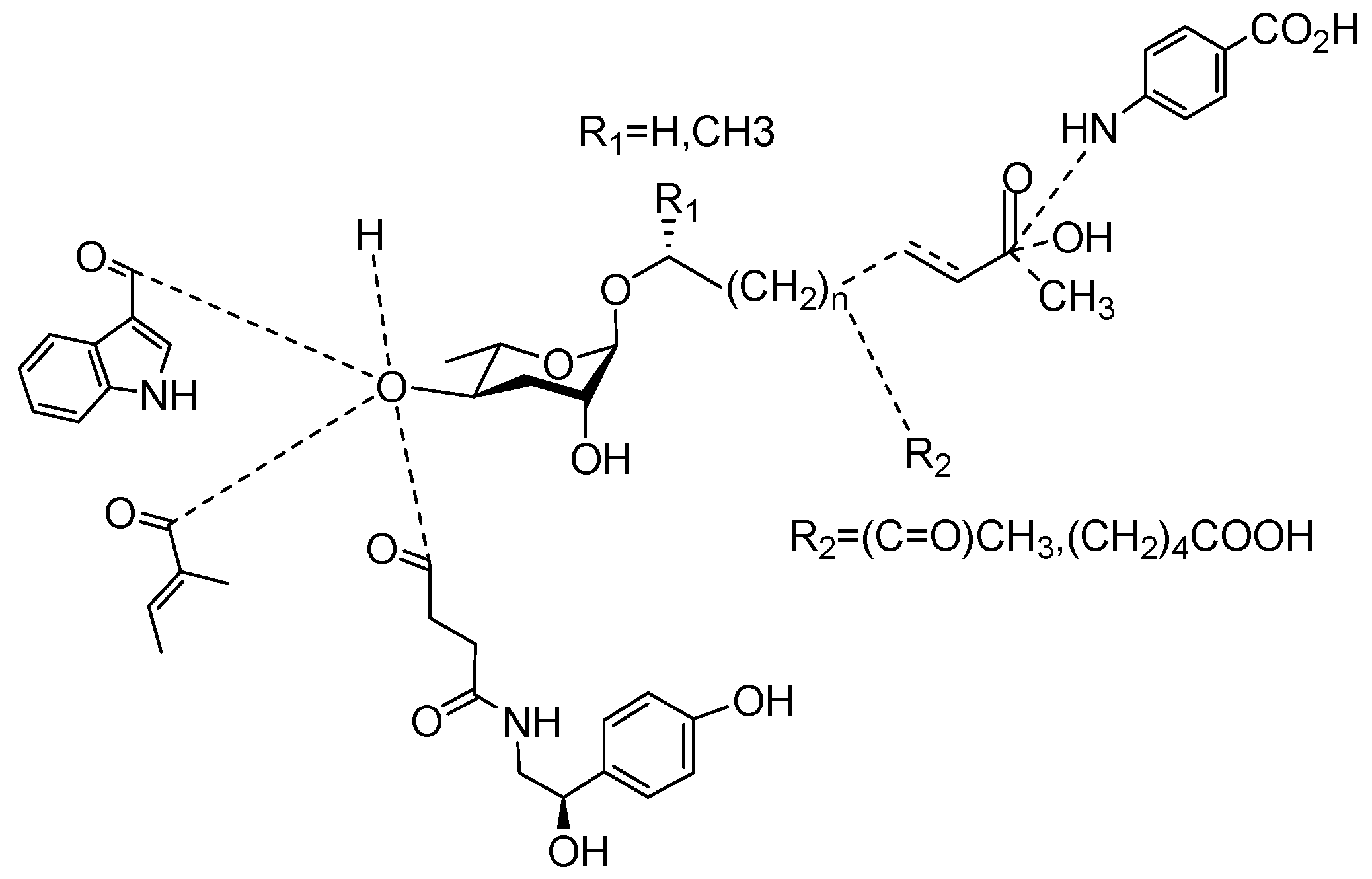
This entry is adapted from the peer-reviewed paper 10.3390/molecules28052409
References
- Kaletta, T.; Hengartner, M.O. Finding Function in Novel Targets: C. elegans as a Model Organism. Nat. Rev. Drug Discov. 2006, 5, 387–398.
- Cassada, R.C.; Russell, R.L. The Dauerlarva, A Post-Embryonic Developmental Variant of the Nematode Caenorhabditis elegans. Dev. Biol. 1975, 46, 326–342.
- Klass, M.; Hirsh, D. Non-Ageing Developmental Variant of Caenorhabditis elegans. Nature 1976, 260, 523–525.
- Schroeder, F.C. Modular Assembly of Primary Metabolic Building Blocks: A Chemical Language in C. elegans. Chem. Biol. 2015, 22, 7–16.
- Riddle, D.L.; Albert, P.S. Genetic and Environmental Regulation of Dauer Larva Development. In C. elegans II; Riddle, D.L., Blumenthal, T., Meyer, B.J., Priess, J.R., Eds.; Cold Spring Harbor Laboratory Press Copyright © 2023; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1997.
- Kim, S.; Paik, Y.K. Developmental and Reproductive Consequences of Prolonged Non-aging Dauer in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2008, 368, 588–592.
- Golden, J.W.; Riddle, D.L. A Pheromone Influences Larval Development in the Nematode Caenorhabditis elegans. Science 1982, 218, 578–580.
- Butcher, R.A. Small-Molecule Pheromones and Hormones Controlling Nematode Development. Nat. Chem. Biol. 2017, 13, 577–586.
- Jeong, P.Y.; Jung, M.; Yim, Y.H.; Kim, H.; Park, M.; Hong, E.; Lee, W.; Kim, Y.H.; Kim, K.; Paik, Y.K. Chemical Structure and Biological Activity of the Caenorhabditis elegans Dauer-Inducing Pheromone. Nature 2005, 433, 541–545.
- Butcher, R.A.; Fujita, M.; Schroeder, F.C.; Clardy, J. Small-Molecule Pheromones that Control Dauer Development in Caenorhabditis elegans. Nat. Chem. Biol. 2007, 3, 420–422.
- Cohen, S.M.; Wrobel, C.J.J.; Prakash, S.J.; Schroeder, F.C.; Sternberg, P.W. Formation and Function of Dauer Ascarosides in the Nematodes Caenorhabditis briggsae and Caenorhabditis elegans. G3 Bethesda 2022, 12, jkac014.
- Srinivasan, J.; Kaplan, F.; Ajredini, R.; Zachariah, C.; Alborn, H.T.; Teal, P.E.; Malik, R.U.; Edison, A.S.; Sternberg, P.W.; Schroeder, F.C. A Blend of Small Molecules Regulates Both Mating and Development in Caenorhabditis elegans. Nature 2008, 454, 1115–1118.
- Shimizu, T.; Sugiura, K.; Sakai, Y.; Dar, A.R.; Butcher, R.A.; Matsumoto, K.; Hisamoto, N. Chemical Signaling Regulates Axon Regeneration via the GPCR-Gqα Pathway in Caenorhabditis elegans. J. Neurosci. 2022, 42, 720–730.
- Park, J.; Oh, H.; Kim, D.Y.; Cheon, Y.; Park, Y.J.; Hwang, H.; Neal, S.J.; Dar, A.R.; Butcher, R.A.; Sengupta, P.; et al. CREB Mediates the C. elegans Dauer Polyphenism Through Direct and Cell-Autonomous Regulation of TGF-β Expression. PLoS Genet. 2021, 17, e1009678.
- Butcher, R.A.; Ragains, J.R.; Kim, E.; Clardy, J. A Potent Dauer Pheromone Component in Caenorhabditis elegans that Acts Synergistically with Other Components. Proc. Natl. Acad. Sci. USA 2008, 105, 14288–14292.
- Park, J.; Choi, W.; Dar, A.R.; Butcher, R.A.; Kim, K. Neuropeptide Signaling Regulates Pheromone-Mediated Gene Expression of a Chemoreceptor Gene in C. elegans. Mol. Cells 2019, 42, 28–35.
- Reilly, D.K.; McGlame, E.J.; Vandewyer, E.; Robidoux, A.N.; Muirhead, C.S.; Northcott, H.T.; Joyce, W.; Alkema, M.J.; Gegear, R.J.; Beets, I.; et al. Distinct Neuropeptide-Receptor Modules Regulate a Sex-Specific Behavioral Response to a Pheromone. Commun. Biol. 2021, 4, 1018.
- Hong, R.L.; Sommer, R.J. Pristionchus pacificus: A Well-Rounded Nematode. BioEssays 2006, 28, 651–659.
- Meyer, J.M.; Baskaran, P.; Quast, C.; Susoy, V.; Rödelsperger, C.; Glöckner, F.O.; Sommer, R.J. Succession and Dynamics of Pristionchus Nematodes and Their Microbiome During Decomposition of Oryctes Borbonicus on La Réunion Island. Environ. Microbiol. 2017, 19, 1476–1489.
- Sommer, R.J.; McGaughran, A. The Nematode Pristionchus pacificus as a Model System for Integrative Studies in Evolutionary Biology. Mol. Ecol. 2013, 22, 2380–2393.
- Bento, G.; Ogawa, A.; Sommer, R.J. Co-Option of the Hormone-Signalling Module Dafachronic Acid-DAF-12 in Nematode Evolution. Nature 2010, 466, 494–497.
- Bose, N.; Ogawa, A.; von Reuss, S.H.; Yim, J.J.; Ragsdale, E.J.; Sommer, R.J.; Schroeder, F.C. Complex Small-Molecule Architectures Regulate Phenotypic Plasticity in a Nematode. Angew. Chem. Int. Ed. Engl. 2012, 51, 12438–12443.
- Falcke, J.M.; Bose, N.; Artyukhin, A.B.; Rödelsperger, C.; Markov, G.V.; Yim, J.J.; Grimm, D.; Claassen, M.H.; Panda, O.; Baccile, J.A.; et al. Linking Genomic and Metabolomic Natural Variation Uncovers Nematode Pheromone Biosynthesis. Cell Chem. Biol. 2018, 25, 787–796.e12.
- Bose, N.; Meyer, J.M.; Yim, J.J.; Mayer, M.G.; Markov, G.V.; Ogawa, A.; Schroeder, F.C.; Sommer, R.J. Natural Variation in Dauer Pheromone Production and Sensing Supports Intraspecific Competition in Nematodes. Curr. Biol. 2014, 24, 1536–1541.
- Werner, M.S.; Claaßen, M.H.; Renahan, T.; Dardiry, M.; Sommer, R.J. Adult Influence on Juvenile Phenotypes by Stage-Specific Pheromone Production. iScience 2018, 10, 123–134.
- Ciche, T.A.; Kim, K.S.; Kaufmann-Daszczuk, B.; Nguyen, K.C.; Hall, D.H. Cell Invasion and Matricide during Photorhabdus luminescens Transmission by Heterorhabditis bacteriophora Nematodes. Appl. Environ. Microbiol. 2008, 74, 2275–2287.
- Ciche, T.A.; Ensign, J.C. For the Insect Pathogen Photorhabdus luminescens, Which End of a Nematode is Out? Appl. Environ. Microbiol. 2003, 69, 1890–1897.
- Ciche, T. The Biology and Genome of Heterorhabditis bacteriophora. WormBook 2007, 20, 1–9.
- Noguez, J.H.; Conner, E.S.; Zhou, Y.; Ciche, T.A.; Ragains, J.R.; Butcher, R.A. A Novel Ascaroside Controls the Parasitic Life Cycle of the Entomopathogenic Nematode Heterorhabditis bacteriophora. ACS Chem. Biol. 2012, 7, 961–966.
- Gomez-Diaz, C.; Benton, R. The Joy of Sex Pheromones. EMBO Rep. 2013, 14, 874–883.
- Wyatt, T.D. Pheromones and Signature Mixtures: Defining Species-Wide Signals and Variable Cues for Identity in Both Invertebrates and Vertebrates. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2010, 196, 685–700.
- Leighton, D.H.; Sternberg, P.W. Mating Pheromones of Nematoda: Olfactory Signaling with Physiological Consequences. Curr. Opin. Neurobiol. 2016, 38, 119–124.
- Simon, J.M.; Sternberg, P.W. Evidence of a Mate-Finding Cue in the Hermaphrodite Nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 1598–1603.
- Pungaliya, C.; Srinivasan, J.; Fox, B.W.; Malik, R.U.; Ludewig, A.H.; Sternberg, P.W.; Schroeder, F.C. A Shortcut to Identifying Small Molecule Signals that Regulate Behavior and Development in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2009, 106, 7708–7713.
- Artyukhin, A.B.; Yim, J.J.; Srinivasan, J.; Izrayelit, Y.; Bose, N.; von Reuss, S.H.; Jo, Y.; Jordan, J.M.; Baugh, L.R.; Cheong, M.; et al. Succinylated Octopamine Ascarosides and a New Pathway of Biogenic Amine Metabolism in Caenorhabditis elegans. J. Biol. Chem. 2013, 288, 18778–18783.
- Choe, A.; von Reuss, S.H.; Kogan, D.; Gasser, R.B.; Platzer, E.G.; Schroeder, F.C.; Sternberg, P.W. Ascaroside Signaling is Widely Conserved Among Nematodes. Curr. Biol. 2012, 22, 772–780.
- Izrayelit, Y.; Srinivasan, J.; Campbell, S.L.; Jo, Y.; von Reuss, S.H.; Genoff, M.C.; Sternberg, P.W.; Schroeder, F.C. Targeted Metabolomics Reveals a Male Pheromone and Sex-Specific Ascaroside Biosynthesis in Caenorhabditis elegans. ACS Chem. Biol. 2012, 7, 1321–1325.
- Choe, A.; Chuman, T.; von Reuss, S.H.; Dossey, A.T.; Yim, J.J.; Ajredini, R.; Kolawa, A.A.; Kaplan, F.; Alborn, H.T.; Teal, P.E.; et al. Sex-Specific Mating Pheromones in the Nematode Panagrellus redivivus. Proc. Natl. Acad. Sci. USA 2012, 109, 20949–20954.
- Shi, C.; Runnels, A.M.; Murphy, C.T. Mating and Male Pheromone Kill Caenorhabditis Males Through Distinct Mechanisms. Elife 2017, 6, 23493.
- Aprison, E.Z.; Dzitoyeva, S.; Angeles-Albores, D.; Ruvinsky, I. A Male Pheromone that Improves the Quality of the Oogenic Germline. Proc. Natl. Acad. Sci. USA 2022, 119, e2015576119.
- Aprison, E.Z.; Ruvinsky, I. Sex Pheromones of C. elegans Males Prime the Female Reproductive System and Ameliorate the Effects of Heat Stress. PLoS Genet. 2015, 11, e1005729.
- Aprison, E.Z.; Ruvinsky, I. Sexually Antagonistic Male Signals Manipulate Germline and Soma of C. elegans Hermaphrodites. Curr. Biol. 2016, 26, 2827–2833.
- Aprison, E.Z.; Ruvinsky, I. Counteracting Ascarosides Act through Distinct Neurons to Determine the Sexual Identity of C. elegans Pheromones. Curr. Biol. 2017, 27, 2589–2599.e3.
- Dong, C.; Dolke, F.; von Reuss, S.H. Selective MS Screening Reveals a Sex Pheromone in Caenorhabditis briggsae and Species-Specificity in Indole Ascaroside Signalling. Org. Biomol. Chem. 2016, 14, 7217–7225.
- Balakanich, S.; Samoiloff, M.R. Development of Nematode Behavior: Sex Attraction Among Different Strains of the Free-Living Panagrellus redivivus. Can. J. Zool. 1974, 52, 835–845.
- Chaudhuri, J.; Kache, V.; Pires-daSilva, A. Regulation of Sexual Plasticity in a Nematode that Produces Males, Females, and Hermaphrodites. Curr. Biol. 2011, 21, 1548–1551.
- Chaudhuri, J.; Bose, N.; Tandonnet, S.; Adams, S.; Zuco, G.; Kache, V.; Parihar, M.; von Reuss, S.H.; Schroeder, F.C.; Pires-daSilva, A. Mating Dynamics in a Nematode with Three Sexes and Its Evolutionary Implications. Sci. Rep. 2015, 5, 17676.
- Riga, E.; Holdsworth, D.R.; Perry, R.N.; Barrett, J.; Johnston, M.R. Electrophysiological Analysis of the Response of Males of the Potato Cyst Nematode, Globodera rostochiensis, to Fractions of Their Homospecific Sex Pheromone. Parasitology 1997, 115 Pt 3, 311–316.
- Srinivasan, J.; von Reuss, S.H.; Bose, N.; Zaslaver, A.; Mahanti, P.; Ho, M.C.; O’Doherty, O.G.; Edison, A.S.; Sternberg, P.W.; Schroeder, F.C. A Modular Library of Small Molecule Signals Regulates Social Behaviors in Caenorhabditis elegans. PLoS Biol. 2012, 10, e1001237.
- Faghih, N.; Bhar, S.; Zhou, Y.; Dar, A.R.; Mai, K.; Bailey, L.S.; Basso, K.B.; Butcher, R.A. A Large Family of Enzymes Responsible for the Modular Architecture of Nematode Pheromones. J. Am. Chem. Soc. 2020, 142, 13645–13650.
- von Reuss, S.H.; Bose, N.; Srinivasan, J.; Yim, J.J.; Judkins, J.C.; Sternberg, P.W.; Schroeder, F.C. Comparative Metabolomics Reveals Biogenesis of Ascarosides, a Modular Library of Small-Molecule Signals in C. elegans. J. Am. Chem. Soc. 2012, 134, 1817–1824.
- Macosko, E.Z.; Pokala, N.; Feinberg, E.H.; Chalasani, S.H.; Butcher, R.A.; Clardy, J.; Bargmann, C.I. A Hub-and-Spoke Circuit Drives Pheromone Attraction and Social Behaviour in C. elegans. Nature 2009, 458, 1171–1175.
- Chute, C.D.; DiLoreto, E.M.; Zhang, Y.K.; Reilly, D.K.; Rayes, D.; Coyle, V.L.; Choi, H.J.; Alkema, M.J.; Schroeder, F.C.; Srinivasan, J. Co-option of Neurotransmitter Signaling for Inter-Organismal Communication in C. elegans. Nat. Commun. 2019, 10, 3186.
- Hussey, R.; Stieglitz, J.; Mesgarzadeh, J.; Locke, T.T.; Zhang, Y.K.; Schroeder, F.C.; Srinivasan, S. Pheromone-Sensing Neurons Regulate Peripheral Lipid Metabolism in Caenorhabditis elegans. PLoS Genet. 2017, 13, e1006806.
- Luo, J.; Portman, D.S. Sex-Specific, Pdfr-1-Dependent Modulation of Pheromone Avoidance by Food Abundance Enables Flexibility in C. elegans Foraging Behavior. Curr. Biol. 2021, 31, 4449–4461.e4.
- Jang, H.; Kim, K.; Neal, S.J.; Macosko, E.; Kim, D.; Butcher, R.A.; Zeiger, D.M.; Bargmann, C.I.; Sengupta, P. Neuromodulatory State and Sex Specify Alternative Behaviors Through Antagonistic Synaptic Pathways in C. elegans. Neuron 2012, 75, 585–592.
- Fagan, K.A.; Luo, J.; Lagoy, R.C.; Schroeder, F.C.; Albrecht, D.R.; Portman, D.S. A Single-Neuron Chemosensory Switch Determines the Valence of a Sexually Dimorphic Sensory Behavior. Curr. Biol. 2018, 28, 902–914.e5.
- Zhang, Y.K.; Sanchez-Ayala, M.A.; Sternberg, P.W.; Srinivasan, J.; Schroeder, F.C. Improved Synthesis for Modular Ascarosides Uncovers Biological Activity. Org. Lett. 2017, 19, 2837–2840.
- Dal Bello, M.; Perez-Escudero, A.; Schroeder, F.C.; Gore, J. Inversion of Pheromone Preference Optimizes Foraging in C. elegans. Elife 2021, 10, 58144.
- Nuttley, W.M.; Atkinson-Leadbeater, K.P.; Van Der Kooy, D. Serotonin Mediates Food-Odor Associative Learning in the Nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 12449–12454.
- Yamada, K.; Hirotsu, T.; Matsuki, M.; Butcher, R.A.; Tomioka, M.; Ishihara, T.; Clardy, J.; Kunitomo, H.; Iino, Y. Olfactory Plasticity is Regulated by Pheromonal Signaling in Caenorhabditis elegans. Science 2010, 329, 1647–1650.
- Zhou, Y.; Loeza-Cabrera, M.; Liu, Z.; Aleman-Meza, B.; Nguyen, J.K.; Jung, S.K.; Choi, Y.; Shou, Q.; Butcher, R.A.; Zhong, W. Potential Nematode Alarm Pheromone Induces Acute Avoidance in Caenorhabditis elegans. Genetics 2017, 206, 1469–1478.
- Greene, J.S.; Brown, M.; Dobosiewicz, M.; Ishida, I.G.; Macosko, E.Z.; Zhang, X.; Butcher, R.A.; Cline, D.J.; McGrath, P.T.; Bargmann, C.I. Balancing Selection Shapes Density-Dependent Foraging Behaviour. Nature 2016, 539, 254–258.
- Hong, M.; Ryu, L.; Ow, M.C.; Kim, J.; Je, A.R.; Chinta, S.; Huh, Y.H.; Lee, K.J.; Butcher, R.A.; Choi, H.; et al. Early Pheromone Experience Modifies a Synaptic Activity to Influence Adult Pheromone Responses of C. elegans. Curr. Biol. 2017, 27, 3168–3177.e3.
- Kaplan, F.; Perret-Gentil, A.; Giurintano, J.; Stevens, G.; Erdogan, H.; Schiller, K.C.; Mirti, A.; Sampson, E.; Torres, C.; Sun, J.; et al. Conspecific and Heterospecific Pheromones Stimulate Dispersal of Entomopathogenic Nematodes During Quiescence. Sci. Rep. 2020, 10, 5738.
- Kaplan, F.; Alborn, H.T.; von Reuss, S.H.; Ajredini, R.; Ali, J.G.; Akyazi, F.; Stelinski, L.L.; Edison, A.S.; Schroeder, F.C.; Teal, P.E. Interspecific Nematode Signals Regulate Dispersal Behavior. PLoS ONE 2012, 7, e38735.
- Hartley, C.J.; Lillis, P.E.; Owens, R.A.; Griffin, C.T. Infective Juveniles of Entomopathogenic Nematodes (Steinernema and Heterorhabditis) Secrete Ascarosides and Respond to Interspecific Dispersal Signals. J. Invertebr. Pathol. 2019, 168, 107257.
- Zhao, M.; Wickham, J.D.; Zhao, L.; Sun, J. Major Ascaroside Pheromone Component asc-C5 Influences Reproductive Plasticity Among Isolates of the Invasive Species Pinewood Nematode. Integr. Zool. 2021, 16, 893–907.
- Meng, J.; Wickham, J.D.; Ren, W.; Zhao, L.; Sun, J. Species Displacement Facilitated by Ascarosides Between Two Sympatric Sibling Species: A Native and Invasive Nematode. J. Pest Sci. 2020, 93, 1059–1071.
- Manosalva, P.; Manohar, M.; von Reuss, S.H.; Chen, S.; Koch, A.; Kaplan, F.; Choe, A.; Micikas, R.J.; Wang, X.; Kogel, K.H.; et al. Conserved Nematode Signalling Molecules Elicit Plant Defenses and Pathogen Resistance. Nat. Commun. 2015, 6, 7795.
- Kong, X.; Huang, Z.; Gu, X.; Cui, Y.; Li, J.; Han, R.; Jin, Y.; Cao, L. Dimethyl Sulfoxide and Ascarosides Improve the Growth and Yields of Entomopathogenic Nematodes in Liquid Cultures. J. Invertebr. Pathol. 2022, 193, 107800.
- Wang, J.; Cao, L.; Huang, Z.; Gu, X.; Cui, Y.; Li, J.; Li, Y.; Xu, C.; Han, R. Influence of the Ascarosides on the Recovery, Yield and Dispersal of Entomopathogenic Nematodes. J. Invertebr. Pathol. 2022, 188, 107717.
- Butcher, R.A.; Ragains, J.R.; Clardy, J. An Indole-Containing Dauer Pheromone Component with Unusual Dauer Inhibitory Activity at Higher Concentrations. Org. Lett. 2009, 11, 3100–3103.
- Ludewig, A.H.; Artyukhin, A.B.; Aprison, E.Z.; Rodrigues, P.R.; Pulido, D.C.; Burkhardt, R.N.; Panda, O.; Zhang, Y.K.; Gudibanda, P.; Ruvinsky, I.; et al. An Excreted Small Molecule Promotes C. elegans Reproductive Development and Aging. Nat. Chem. Biol. 2019, 15, 838–845.
- Jaffe, H.; Huettel, R.N.; Demilo, A.B.; Hayes, D.K.; Rebois, R.V. Isolation and Identification of a Compound from Soybean Cyst Nematode, Heterodera glycines, with Sex Pheromone Activity. J. Chem. Ecol. 1989, 15, 2031–2043.
- Meyer, S.L.; Johnson, G.; Dimock, M.; Fahey, J.W.; Huettel, R.N. Field Efficacy of Verticillium lecanii, Sex Pheromone, and Pheromone Analogs as Potential Management Agents for Soybean Cyst Nematode. J. Nematol. 1997, 29, 282–288.
