Pulmonary hypertension (PH) is a pathophysiological disorder that may involve various clinical conditions and may be associated with numerous respiratory and/or cardiovascular diseases. Exercise echocardiography can unmask exercise PH, detect the early stages of left ventricular diastolic dysfunction, and, therefore, differentiate between pre- and post-capillary PH. Regardless of the underlying aetiology, a developed PH is associated with increased mortality. Parameters of overt right ventricle (RV) dysfunction, including RV dilation, reduced RV ejection fraction, and elevated right-sided filling pressures, are detectable with resting echocardiography and are associated with worse outcome.
1. Diagnostic Role
In patients with exertional dyspnoea and suspected PH due to HFpEF, there is a possibility to unmask early stages of LV diastolic dysfunction to detect increased LV filling pressures during exercise and, therefore, to differentiate between pre- and post-capillary PH.
As in resting echocardiography, the increase in the E/e′ ratio during exercise is suggestive of elevated LV filling pressures. However, studies comparing haemodynamic data acquired by echocardiography and by RHC during exercise are limited [
3,
25]. Even though the E/e′ ratio during exercise had only a moderate correlation with directly invasively measured PAWP (r = 0.57;
p < 0.001), adding the peak exercise E/e′ ratio to the ESC proposed algorithm of diastolic dysfunction improved sensitivity (up to 90%) and can be used to rule out post-capillary PH [
10]. Using low-level exercise (20 W) seems to be a good alternative, as E/e′ at 20 W could reliably predict normal PAWP during exercise (AUC: 0.77;
p < 0.01) [
29]. The authors proposed a cut-off of 12.4 for E/e′ at 20 W (specificity 83%, sensitivity 75%). However, these studies comprised only healthy controls and patients with HFpEF. There is only one study that tested the echocardiographic mPAP/CO ratio to identify patients with abnormal pulmonary vascular response to exercise [
30]. In a study group of healthy subjects and mainly patients with chronic thromboembolic pulmonary hypertension, mPAP/CO via exercise stress echocardiography of 3.2 mmHg/L/min was identified as the most favourable threshold. Of note, this cut-off is perfectly in line with the proposed cut-off obtained by RHC.
In spite of the above-mentioned data, the diagnostic value of the stress tests during echocardiography to distinguish between PH subtypes is currently uncertain due to the lack of prospective data, especially regarding its use to identify cases of combined post- and pre-capillary PH. Based on the data from the literature, exercise echocardiography is considered abnormal if the average E/e′ ratio at peak stress increases to ≥15, with or without a peak TR velocity >3.4 m/s (
Figure 1) [
10,
11,
12]. An increase in TR velocity only should not be used to diagnose post-capillary PH because it might be a normal hyperdynamic response to exercise with increased pulmonary blood flow in the absence of LV diastolic dysfunction [
31].
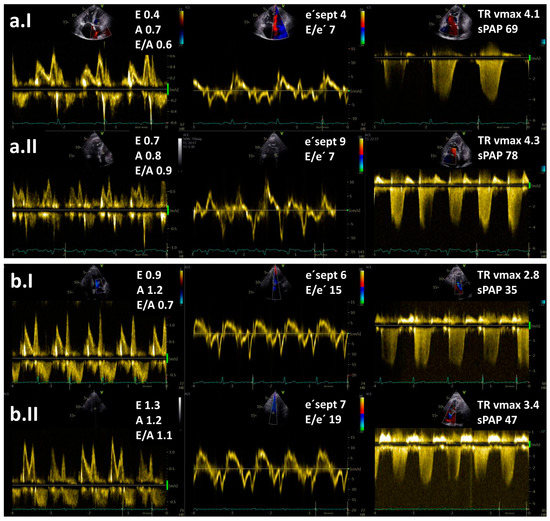
Figure 1. Resting (I) and exercise stress echocardiography (II) in two patients with pulmonary hypertension (PH): (a) a patient with pre-capillary PH and (b) a patient with post-capillary PH. Maximal tricuspid regurgitation velocity (TR vmax) is elevated in both patients. Note an increase in E/e′ ratio at low-level exercise from 15 to 19 in a patient with post-capillary PH but no increase in a patient with pre-capillary PH. Legend: sPAP—systolic pulmonary arterial pressure.
Exercise stress echocardiography could be extremely useful as an effective gatekeeper to the RHC for patients with exertional dyspnoea of unknown aetiology and normal resting echocardiographic results and also for identifying patients with a high risk for developing PH. It has been demonstrated that exercise stress echocardiography can distinguish between noncardiac and cardiac causes of unexplained dyspnoea [
10,
32]. The diagnostic value of exercise stress echocardiography was also evaluated in asymptomatic relatives of patients with idiopathic and familial PAH [
33]. Hypertensive response to exercise, defined by TR velocity > 3.1 m/s, was more often present in relatives of PAH patients than in control subjects. Additionally, exercise stress echocardiography is considered to be reasonable, especially in patients with connective tissue disease [
34]. It has been reported that up to 50% of this patient population with normal resting mPAP had an abnormal increase in mPAP during exercise. In patients with systemic sclerosis, PH was confirmed by RHC in 81% of patients with positive exercise stress echocardiography [
35]. Moreover, exercise stress echocardiography could unmask exercise PH in patients with systemic sclerosis and baseline echocardiographic PAP within the grey zone [
36]. It is important to note that in both studies, authors used a definition of exercise PH, which is not in line with nowadays valid definition (an increase of 20 mmHg over the resting sPAP or sPAP > 50 mmHg was considered as a positive test result). However, the clinical value of exercise PH identified by exercise stress echocardiography remains uncertain because of the lack of validated criteria and prospective confirmatory data [
1]. Therefore, data from exercise stress echocardiography are not sufficient to be a substitute for invasive haemodynamic data under all circumstances, especially if a therapeutic decision depends on the results [
9].
2. Prognostic Role
Regardless of the underlying aetiology, the developed PH is associated with worsening symptoms and substantially increased mortality [
37]. Even though the detection of exercise PH via exercise stress echocardiography is considered an early and mild phase of PAH [
38], patients with exercise PH already had worse outcomes than subjects without exercise PH [
39].
The survival of PH patients depends on the capability of the RV to adapt to chronically elevated PAP [
40]. Over time, adaptive concentric RV hypertrophy with preserved RV function can evolve into RV dilatation and systolic dysfunction [
41,
42]. RV function is a major determinant of functional capacity and prognosis when RV afterload is elevated [
43,
44,
45]. Echocardiographic measures of RV function that are independent predictors of mortality in PH include the tricuspid annular plane systolic excursion (TAPSE < 18 mm [
46,
47,
48]), RV fractional area change (FAC < 35% [
49,
50]), peak systolic tricuspid lateral annular velocity (S’ < 9.7 cm/s [
51]) and Tei index (>0.40 by pulse Doppler or >0.55 by tissue Doppler [
52]). Conventional 2-dimensional echocardiographic evaluation of the RV is difficult due to the complex 3-dimensional (3D) anatomical shape of the RV. This limitation can be overcome with 3D echocardiography and/or cardiac magnetic resonance [
53,
54] and recently, an increased 3D RVESVi has been shown to correlate with increased mortality [
55].
However, these parameters all fail to identify occult RV dysfunction in patients with PH [
56] as they reflect already established RV dysfunction. Subtle RV dysfunction could possibly be recognised by the use of advanced echocardiographic techniques, such as strain/myocardial deformation and myocardial work [
57,
58,
59]. Previous studies demonstrated that RV longitudinal strain was a powerful predictor of survival in patients with PH and provided incremental prognostic value over conventional clinical and echocardiographic variables [
60,
61].
Additionally, the assessment of RV contractile reserve via RV–pulmonary arterial (PA) coupling shows promising results in detecting subclinical RV systolic dysfunction [
56,
62,
63,
64,
65,
66]. Gold standard measurement of RV–PA coupling involves conductance catheter measurement of “multi-beat” RV end-systolic elastance (Ees), a method that remains costly, impractical and clinically challenging [
62]. However, new echocardiographic indices, such as the TAPSE/sPAP ratio [
45,
67,
68,
69] and RV free wall longitudinal strain/sPAP [
59], are tightly linked to RV–PA coupling and are associated with outcomes in patients with PH.
A possible non-invasive measure of the RV contractile reserve using exercise stress echocardiography was first proposed by Grünig et al. They demonstrated that an exercise-induced increase in sPAP was a measure of the RV contractile reserve and was an independent prognostic factor in patients with pre-capillary PH [
70]. A lower sPAP increase may reveal an impaired ability of the RV to adapt to pulmonary load and exercise and to further increase pressure and pulmonary blood flow. Similarly, an initial steep increment in PAP during exercise followed by a plateau with a linear pattern was associated with decreased exercise capacity and survival in patients with heart failure [
17]. Echocardiographic studies focused only on the peak exercise sPAP or the peak change in sPAP [
17,
70]; however, it would be preferable to interpret exercise PAP pattern relative to the increase in blood flow (PAP/CO ratio). Invasively obtained haemodynamic data clearly showed that high mPAP/CO during exercise was associated with impaired survival in a heterogeneous group of different PH phenotypes [
5,
14]. Echocardiographic studies analysing mPAP/CO are limited, but initial results are very promising. A disproportionate increase in mPAP/CO slope during exercise was independently associated with adverse clinical outcomes in patients with HFpEF (
Table 1) [
71], and this parameter had an incremental value even in patients with preserved RV-PA coupling at rest.
Other authors assessed RV contractile reserve based on echocardiographic parameters of RV systolic function (e.g., change in TAPSE, change in RV FAC and change in S’) (
Table 1) [
72,
73]. The magnitude of the increase in all three parameters was significantly lower in patients with pre-capillary PH than in healthy controls [
72,
73]; however, no prognostic data have been available for these parameters. Ireland et al. prospectively studied RV contractile reserve in PH patients who underwent cardiac magnetic resonance, echocardiography, and supine invasive cardiopulmonary exercise testing with concomitant RV pressure-volume catheterisation. RV contractile reserve during exercise, measured by Ees during exertion, was associated with an elevation in PAP but the preservation of RV volumes. The lack of RV reserve, on the other hand, was tightly coupled with acute RV dilation during exercise [
62]. RV ejection fraction during exercise was shown to be a robust surrogate for RV contractile reserve (
Table 1), and it best predicted occult RV dysfunction among a variety of resting and exercise RV measures and was also associated with clinical worsening [
62]. Therefore, echocardiographic parameters of RV contractile reserve and exercise stress echocardiography could be useful for follow-up assessment, especially to identify PH patients at high risk [
70].
Table 1. Non-invasive measures of the right ventricle (RV) contractile reserve during exercise stress echocardiography.
This entry is adapted from the peer-reviewed paper 10.3390/life13061385