Milk whey proteins are one of the most valued constituents due to their nutritional and techno-functional attributes. Whey proteins are rich in bioactive peptides, possessing bioactive properties such as being antioxidant and antihypertensive as well as having antimicrobial activities, which, when ingested, confers several health benefits. These peptides have the potential to be used as an active food ingredient in the production of functional foods. In addition to their bioactivities, whey proteins are known to possess enhanced functional attributes that allow them to be utilized in broad applications, such as an encapsulating agent or carrier materials to entrap bioactive compounds, emulsification, and in edible and active packaging.
- bioactive
- functional
- encapsulation
- formulation
- whey proteins
1. Introduction
Bovine milk is one of the most nutritious foods and is widely used for human consumption. It is one of the rich sources of nutrients that have several biological properties that impact the biochemical processes in our body, influences the development and functioning of specific organs, and protection from diseases. Milk provides a wide range of biologically active components such as bioactive proteins and peptides, oligosaccharides, immunoglobulins, and fats/lipids that protect against pathogens and illnesses on regular consumption.
Milk can be sourced from several milch animals including, cow, buffalo, goat, and sheep. Bovine milk contains approximately a total protein of 3.5%, fats, and essential vitamins, that support growth and development [1]. It is a natural and rich source of well-balanced nutrients that show a diverse range of bio functional properties. These properties are because of the presence of milk proteins/peptides, which support infant development, its growth, and confers health benefits beyond basic nutrition [2]. Besides, proteins extracted from milk are well characterized for their multiple functional characteristics and are utilized by several industries in food applications. The milk protein system is constituted majorly by two kinds of proteins: approximately 80% (w/w) casein, which is generally extracted from skim milk through precipitation using either an acid (isoelectric precipitation) or enzymes (rennet coagulation) and 20% whey, which is a leftover byproduct after the casein is extracted [3]. Majorly whey portion of milk contains five fractions which altogether make up 85% of whey protein. These fractions include α-lactalbumin, β-lactoglobulin, glycomacropeptide, immunoglobulins, protease peptone, and serum albumin whereas the casein portion of milk contains β-casein, αs1-casein, αs2- casein, and κ-casein [4].
Proteins are macronutrients when consumed as supplements may exhibit favorable effects on growth metabolism and health [5][6]. Several reports show that protein deficiency is one of the major health concerns globally [7], and considering this condition, the introduction of dietary protein-rich supplements is of utmost importance. Some of the by-products from agricultural industries like, fruit pomace [8], soy extract [9], cereal brans [10], and milk whey [11], are increasingly getting popular as food ingredients with healthy components. This review focuses on exploiting the bioactive and functional properties of milk whey proteins.
Whey is a yellow-green colored liquid portion of milk, also called cheese serum is obtained after separation of the curd, during coagulation of milk using proteolytic enzymes or acids [12]. It was considered as a major dairy waste for decades because of the disposal issues related to its high biological oxygen demand and high organic matter [13]. However, nowadays whey proteins are recognized as a potential source of nutrients and are exploited for its bioactive ingredients. Because of its high nutritional composition, it is used in several commercial food product applications and is significantly associated with the dairy industry. Generally, fresh liquid whey from cheese-making is composed of 94.2% water and 50% of the total solids of which 0.8% is whey proteins, 0.5% is minerals, 0.1% is fat and 4.3% is lactose, which is the main constituent [14]. However, the composition and the characteristics of whey may vary with the type of cattle, the diet of the animal, milk from which it is produced, processing techniques used, and other environmental factors [15]. Whey proteins are a form of globular proteins, containing a considerable number of α-helix patterns with evenly distributed hydrophilic and hydrophobic, and acidic and basic amino acids along their polypeptide chain [16]. The major constituents of whey proteins include α-Lactalbumin (α-LA); β-Lactoglobulin (β-LG), bovine serum albumin (BSA), immunoglobulins (IG), bovine lactoferrin (BLF), bovine lactoperoxidase (LP), and minor amounts of glycomacropeptide (GMP). The composition of each constituent is shown in Table 1. However, the whey protein composition will vary based on the whey type i.e., sweet whey or acid whey, type of milk i.e., bovine, ovine or caprine, type of cattle feed, lactation stage, and the type of processing. Whey, acidic in nature will have a pH of approx. 5.1 and is generally produced through direct acidification whereas sweet whey has a pH of around 5.6 is produced through rennet-coagulation particularly during the cheese-making process [17].
Table 1. Whey protein constituents and its composition a.
|
Whey protein constituent |
Concentration (g/L) b, e
|
Molecular weight in kDa c, d |
Number of amino acid residues c |
References |
|
α-Lactalbumin |
1.2 |
14175 |
123 |
a [3] b [18] c [19] d [20] e [21] |
|
β-Lactoglobulin |
1.3 |
18277 |
162 |
|
|
Bovine serum albumin |
0.4 |
66,267 |
582 |
|
|
Immunoglobulins (A, M and C) |
0.7 |
25,000 (light chain) and 50,000-70,000 (heavy chain) |
- |
|
|
Bovine Lactoferrin |
0.1 |
80,000 |
700 |
|
|
Glycomacropeptide |
1.2 |
6700 |
64 |
|
|
Bovine Lactoperoxidase |
0.03 |
70,000 |
612 |
2. Whey protein derivatives: Concentrates, Isolates and Hydrolysates
With the rising popularity of healthy eating, there is a worldwide demand for food products formulated with high protein [22]. The daily average protein intake for a sedentary person should be 0.8g per kg of body weight per day (g/kg/day) [23]. This amount of protein is required to maintain a positive nitrogen balance and healthy metabolic function in the body. There are various forms of supplemental proteins available such as egg, soy, hemp, whey, casein. Among these, milk whey contains the maximum concentration of amino acids that are readily available and easy to digest, making it effectively incorporate into body cells [24].
Besides, milk whey proteins are recognized as healthy ingredients because of their several advantages associated with their regular intake, including appetite control, exercise recovery, and promoting satiety [25]. In recent years, several applications of membrane filtration have enabled the use of different whey protein components as food additives. Using selective membranes, after the milk is coagulated the whey protein is extracted in two main forms: whey protein concentrates (WPCs) (having ~34-89% protein) and whey protein isolates (WPIs) (having at least 90% protein) [26][27]. Passing the whey proteins through various processing treatments leads to the formation of whey products (Figure 1) with different qualitative and quantitative protein profiles, and minerals, lipids, and sugars. Application of selective membranes to fractionate whey proteins include ultrafiltration (UF) to concentrate proteins or the use of the diafiltration (DF) method to exclude the molecular compounds like minerals, lactose, and other low weight components. This leads to the production of whey protein concentrates (WPC) [24]. It is the most concentrated form of protein supplement, that has high calories and contains all the macro and micro-nutrients derived from the manufacturing process. However, based on the protein concentration, it can be of several types like WPC of 35%, 50%, 65%, and 80% (w/w) protein. When most of the components are removed i.e., the whey undergoes an additional purification step to eliminate or minimize the extraneous carbohydrates and fats to obtain a protein threshold of 90% (w/w), it is referred as whey protein isolate (WPI). Though being a high-quality protein, the disadvantage of an isolated form of whey protein is that the purification leads to the elimination of some of the important micro-nutrients and protein fractions like lactoferrins, β-lactoglobulins, and immune-globulins.
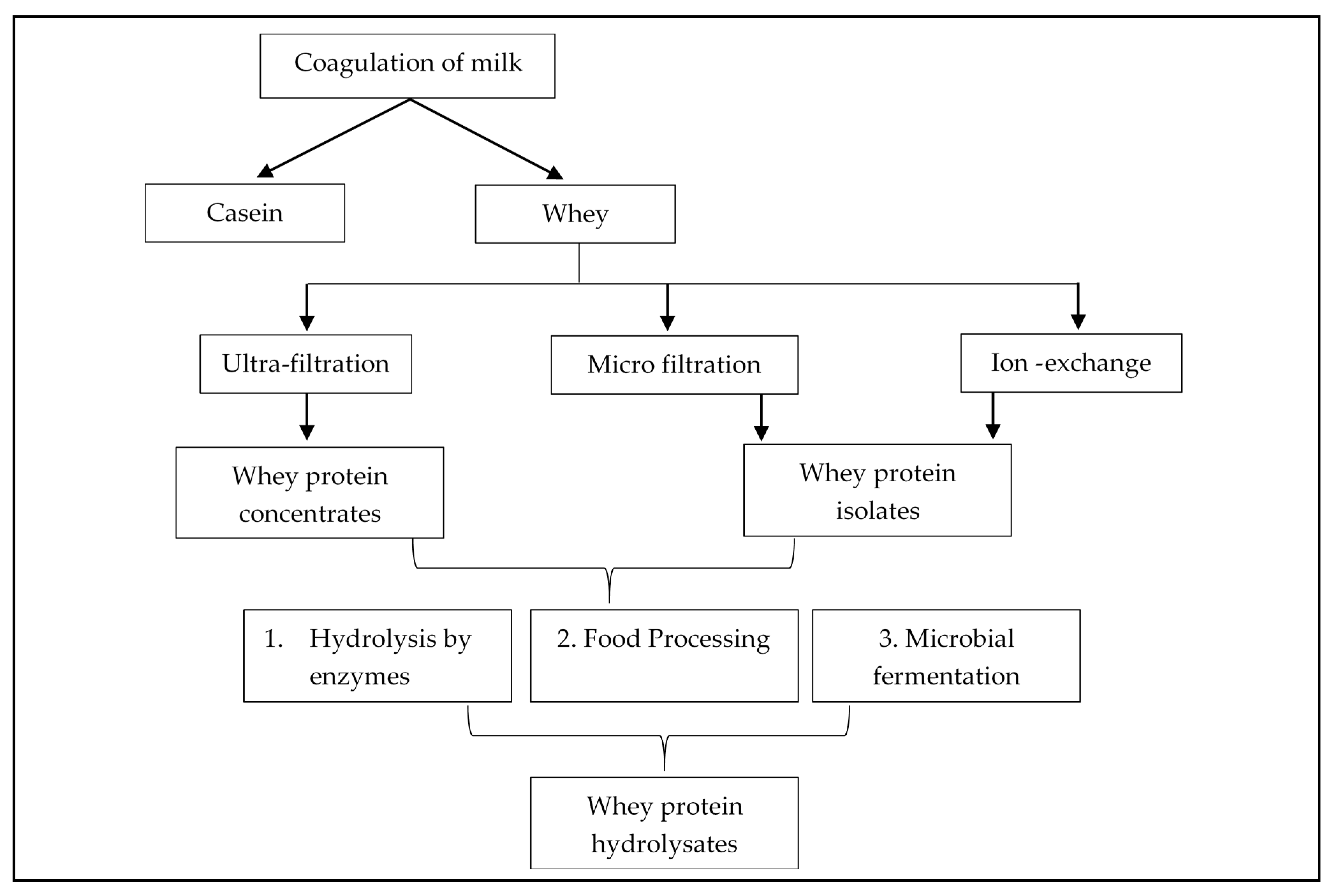
Figure 1. Production of whey protein derivatives
The concentrates and isolates are composed of large intact protein structures, hence, during digestion, the enzymes in our digestive tract break down these proteins, targeting the amino acid bonds, to generate smaller peptides with amino acid sequences. To facilitate this process and make the protein absorption faster, the manufacturers pre-digest the protein to produce protein hydrolysates.
When whey protein concentrates or isolates are treated with acids, enzymes, or heat, the intact form of protein breaks down into peptides and amino acids leading to the formation of whey protein hydrolysates (WPH). These pre-digested forms of whey protein are effectively absorbed in the gut, and hydrolysates that are produced through enzymatic hydrolysis using protease enzyme, contains the identical amino acid composition to that of concentrate and isolate, thus on ingestion, can rapidly increase the amino acid concentration in the plasma as compared to intact forms of protein [28]. The final composition of the hydrolysate largely depends on the type of process implied to break the proteins, the type of enzymes used, reaction or hydrolysis conditions applied, and the number of amino acid bonds that are targeted and broken. Therefore, the degree of hydrolysis is measured to determine the release of the amino acids. The greater the degree of hydrolysis, the smaller the amino acids per peptide, resulting in the generation of more bitter peptides [29]. However, all these forms of proteins are enriched with several benefits and used as food additives to exhibit biological properties.
3. Biological properties of whey proteins associated with bioactive peptides
Isolated protein fragments, containing 2 to 20 amino acid residues, that influence health by delivering beneficial effects on body functions are referred to as bioactive peptides. Bioactive peptides can be isolated from different food proteins either through gastrointestinal digestion or through fermentation using proteolytic lactic acid bacteria. Depending on their amino acid chains, bioactive peptides, on ingestion may significantly affect the body functions related to the digestive, immune, cardiovascular, or nervous system. These amino acid sequences are specific to their actions in delivering health effects. For example, peptides exhibiting antioxidative, antimicrobial, ACE inhibition, and immunomodulation will possess specific known peptide sequence [30][31][32][33][34]. Some of these peptides also exhibit multi-functional activities [35]. Hence, these bioactive peptides are recently being used in several food applications for the development of pharmaceutical, nutraceutical, and functional foods [36].
Biological properties of whey proteins (Figure 2) are widely recognized and have been increasingly exploited in scientific research studies and food applications by various industries. β-lactoglobulins contribute to 50% of the whey protein, which helps to bind minerals like zinc and calcium. It also has partial sequence homology to retinol-binding proteins. α-lactalbumin, on the other hand, is strongly advised to be added in the infant formulas or into foods to develop protein rich dietary intakes [37]. Serum albumin can bind fatty acids and immunoglobulins like IgA, IgM, IgG1, and IgG2, which helps to develop passive immunity in consumers [38]. Other protein fractions like lactoferrin is an iron-binding protein that increases the iron absorption in the digestive tract to inhibit enteric microorganisms and promote the growth of desirable microorganisms. It also modulates the immune system and is considered as the major non-specific disease resistance factor in the mammary gland. Lactoferricin is a peptide derived from Lactoferrin is used against intestinal pathogens. Lactoperoxidase is an enzyme with antibacterial properties that is used as a natural preservative to control acid development in milk during refrigerated storage [39]. Whey proteins are a rich source of essential amino acids like cysteine, branched-chain amino acids like leucine, isoleucine, and valine, and in bioactive peptides [40]. Leucine plays an important role in regulating the synthesis of skeletal muscle protein and is 50-75% higher in whey proteins as compared to other sources [41]. It is also high in Sulphur rich amino acids i.e., cysteine which is a precursor of glutathione [42]. Glutathione is a non-enzymatic thiol obtained from the diet, that acts as an antioxidant. It helps to protect from diseases by reducing the antioxidative stress and regulating the cellular processes [43]. Glycomacropeptide (12%), released during rennet coagulation of cheese, is a casein-derived whey peptide that has many health benefits including satiety and phenylketonuria management [44]. Specific biological functions of the whey protein components are given in Table 2.
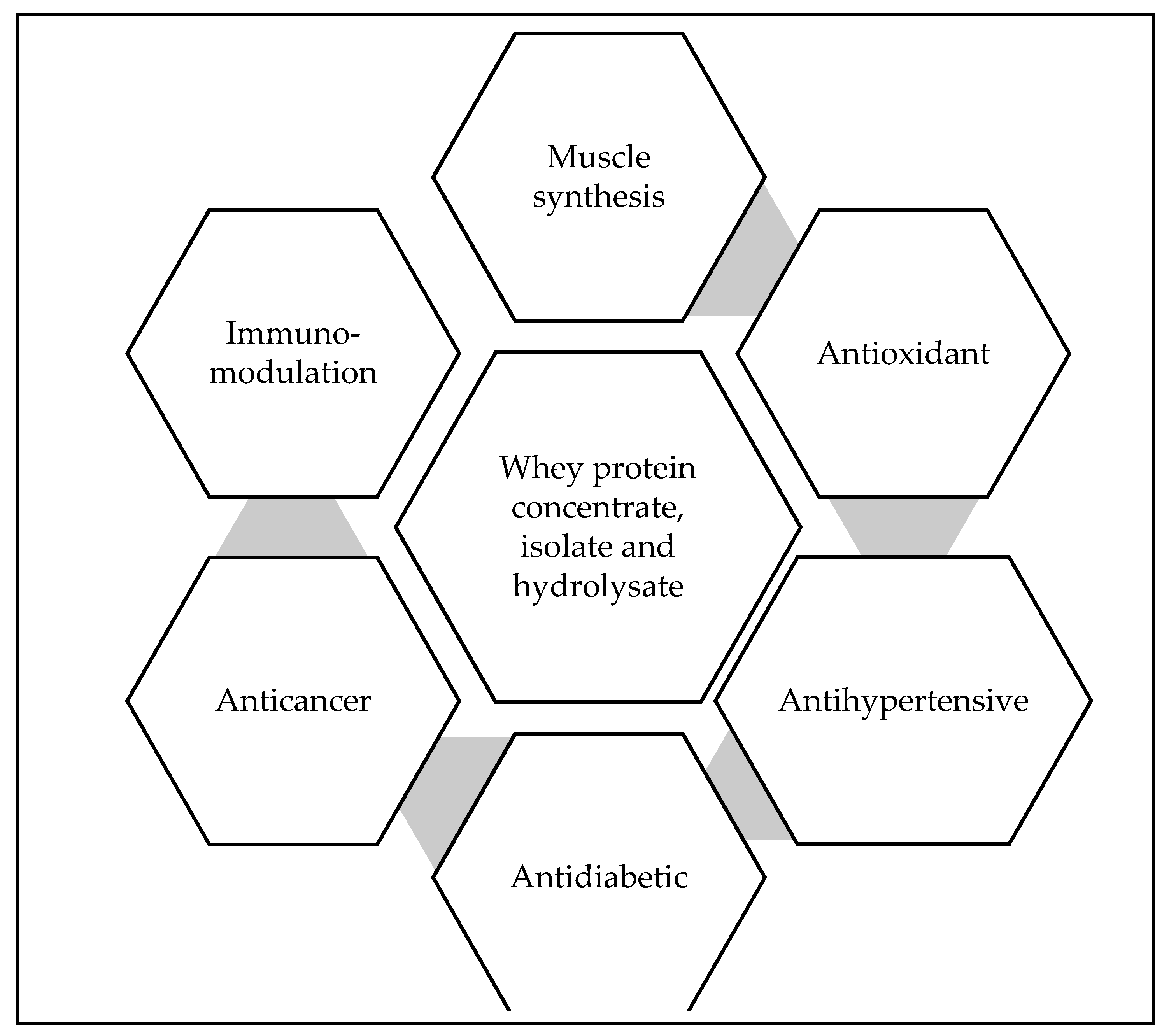
Figure 2. Biological properties of the whey protein derivatives.
Table 2. Biological activities of the major whey protein constituents based on Madureira et al., 2007 [3]
|
Whey protein constituent |
Biological activities |
References |
|
α-Lactalbumin |
Anticancer activity Lactose metabolism and synthesis Treatment of chronic stress-induced disease |
[18] [45]
[46] |
|
β-Lactoglobulin |
Transporter of retinol, fatty acids, palmitate, vitamin D and cholesterol Increase in pregrastic esterase activity Mammary gland phosphorus synthesis and metabolism Passive immunity transfer |
[50]
[51]
[52] |
|
Bovine serum albumin |
Bind fatty acids Anti-mutagenic activity Anti-cancer activity Immune system modulation through passive immunity |
[53] [54] [55] |
|
Immunoglobulins (A, M and C) |
Antimicrobial activity Antifungal activity Opioid activity |
[58] [59] [60] |
Bioactive properties of the peptides are determined based on their amino acid sequence and molecular weight. Mostly the peptides are of short-chain length with 2 to 6 amino acid sequences, however, some peptides with high molecular weight are made of 30 amino acids. Hence, to isolate these peptides, firstly they can be passed through an ultra-filtration membrane of varying molecular weight such as 10kDa, 5kDa, or 3kDa. Another technique that has been commonly used for separating and purifying these bioactive peptides is High-Performance Liquid Chromatography (HPLC). Other methods such as Sodium dodecyl sulfate-polyacrylic gel electrophoresis (SDS-PAGE) and ultra-centrifugation, are also implied for the characterizing the protein and identifying the amino acid composition of the peptides. Recently, several other methods like electrospray ionization (ESI), mass spectrometry (MS), matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) are being used to isolate, identify, and characterize the bioactive peptides. Among these methods, mass spectrometry has been used to generate the peptide profile and determine the molecular mass and amino acid sequences of the protein hydrolysates.
4. Functional Properties of Whey Proteins
Functional properties of proteins refer to the physicochemical properties that play an important role in imparting a specific behavior and performance to proteins when added in food systems. The properties of whey proteins include thermal stability, hydration, gelling, and emulsification properties, which influence the final quality of foods. These properties vary with the interaction among proteins or with other food components and are strongly affected during preparation, processing, storage, and consumption of the foods. Some of the processing conditions and extrinsic and intrinsic parameters that influence the functionalities of whey proteins are shown in Table 3. Whey proteins and derivatives vary in their composition and, hence, possess different functional properties. As a result, they are used in different food applications.
Table 3. Processing conditions, extrinsic and intrinsic parameters affecting the functional properties of whey proteins (Sourced from [61])
|
Processing conditions |
Heating Acidification Counter ions Ionic strength Reducing conditions Drying Storage conditions Modifications related to physical, chemical, enzymatic, and genetic |
|
Extrinsic parameters |
Temperature pH Oxidation-reduction potential Salts or ions Water Carbohydrates Lipids, Gums, Surfactants Tannins |
|
Intrinsic parameters |
Protein composition Monomeric oligomeric Protein blends Rigidity/flexibility Hydrophobicity or hydrophilicity Surface charge Bound flavor ligands |
5. Current Applications of Whey Proteins and Its Derivatives
5.1. Role of Whey Proteins and Derivatives as Food Ingredients
In food applications, whey proteins and derivatives are gaining attention due to their immense benefits owing to several functionalities, including gelation, foaming, emulsification, solubility, and thermal properties. The addition of the whey proteins is known to improve the food sensory quality and enhance the texture. For example, whey proteins have been previously added to foods such as yogurt, bakery foods, energy bars, pasta, and beverages to influence the overall quality and nutrition of the foods. A study reported the effect of adding a complex of non-heat-treated whey protein and high methoxyl pectin in low-fat yogurt [62]. The whey protein acted as a good fat-replacer and texturing agent for the yogurt. Another study showed the ability of the whey proteins to stabilize emulsions and improve the overall texture when added into whole-fat yogurt prepared from skim milk powder. When the droplets merger was used, it yielded whey protein agglomerates with a high molecular weight and reduced emulsifying capacity; however, when passed through a high-pressure homogenizer at 20–100 MPa, it yielded a more stable emulsion [63]. In a study, the effect of the addition of the milk-protein ingredients on the microstructure of probiotic yogurt (prepared with a combination of commercial starter culture and Bifidobacterium lactis Bb12) was analyzed during a 28-day-period refrigerated storage [64]. One sample was added with sodium caseinate at the level of 2% and the other was added with a whey protein concentrate at 2%. It was reported that the addition of sodium caseinate transformed the firmness, adhesiveness, and the overall viscosity of the product, whereas the product added with whey protein demonstrated an improved water holding capacity, viscous texture, and low syneresis as compared to the caseinate. Whey protein in combination with a plant protein was added into a date bar and the nutritional profile was optimized applying a response surface method (RSM) targeting the school children [65]. An addition of 6.05% of whey protein concentrate (WPC) was found to be ideal. Several research studies are still ongoing to utilize whey proteins and their derivatives to develop nutraceutical and functional foods.
5.2. Benefits of Combination of Whey Proteins and Derivatives with Other Supplements
Extensive hydrolysis of whey proteins using enzymes can lead to the formation of bitter peptides, reducing their acceptability in food applications. Enzymatic hydrolysis breaks down the protein fractions like α-lactalbumin, β-lactoglobulin, and serum albumin to generate whey protein hydrolysates containing bitter peptides. This bitter taste of the peptides are often masked using various inhibitors and some of these inhibitory compounds include sucralose, fructose, adenosine 5′ monophosphate, sucrose, adenosine 5′ monophosphate disodium, monosodium glutamate, sodium chloride, sodium gluconate, and sodium acetate [66]. Several techniques involve identifying the bitter peptides and removing them to improve their sensory properties. Liu and coworkers identified four peptides contributing to bitterness in a whey protein hydrolysate. Fractionation techniques (ultra-filtration and chromatography) were used followed by LC-TOF-MS/MS (Liquid chromatography-time of flight-mass spectrometry) to identify the peptides and the constituent amino acids [67]. Gad and his team reported an improvement in the antioxidant and metal chelating activities of the whey protein concentrate (WPC) when supplemented with freshwater algae, spirulina, in both in vitro and in vivo subjects using rat models [68].
5.3. Role of Whey Proteins and Derivatives as Encapsulating Agents and Coating Materials
As consumers become more health-conscious, they are looking for natural ingredients rich in nutrients inside their foods and beverages [69]. Hence, processors are responding to this trend by continually incorporating healthy ingredients in foods or as supplements. Recently, bioactive compounds (e.g., vitamins, antioxidants, minerals and ions, flavor, aroma compounds, lycopene, fats or enzymes or bacterial cells like probiotic microorganisms) have emerged as functional ingredients, leading to the production of novel formulations and value-added foods [70]. However, there are several challenges faced during the application of these bioactive molecules [71]. As a result, to overcome these challenges and considering the increasing demand for value-added novel ingredients in food, food manufacturers started implementing the process of encapsulation [72]. These wide ranges of active compounds can be encapsulated or packaged in a carrier material composed of whey protein. The process of encapsulation involves the incorporation of any solid, liquid, or gaseous materials, including ingredients, enzymes, cells, or other molecules in different carrier materials to produce capsules of varying sizes [69]. This facilitates transporting the agents at the delivery site and based on the strength of the carrier material, the core agents get released at various intervals. Besides, entrapping in a whey protein gel is known to reduce rancidity issues and augment stability. For instance, fortifying foods with iron presents numerous difficulties, and to address this problem, whey protein isolate was used, by utilizing its gelling properties. The isolate was exposed to cold-set gelation to form a matrix, and subsequently iron was entrapped in it in the presence of ascorbate [73]. This led to improving the encapsulation efficiency of the whey protein to recover more iron and improve the in vitro bio-accessibility from 10% to 80%. The use of ascorbate contributed to strengthening the whey protein gel, which led to increased recovery of iron and improved its release characteristics. Similarly, a whey protein concentrate was used as an encapsulant to entrap folic acid. A favorable interaction between the folic acid and the protein matrix was observed, making it a suitable matrix for incorporating vitamins. When compared with a polymer (commercial resistant starch), the WPC capsules imparted a higher stability to folic acid [74]. Whey protein encapsulants can also be formed in combination with other carrier materials, such as carbohydrates and fats. A study demonstrated the efficiency of the whey protein isolate nanoparticle when combined with and without methoxyl pectin [75]. The results showed improved resistance to homogenization and overall stability of the encapsulants formed with pectin. Even during storage at pH 3, the nanoparticle suspension displayed higher interfacial pressures as compared to encapsulants without pectin. Such encapsulants can be potentially used as effective surfactants. An important benefit of the encapsulation process is to prevent the reaction of the core ingredient with other food components, like in the case of essential oils [76]. Besides containing several compounds like phenols, alcohols, esters, ketones, and aldehydes, essential oils exhibit a wide spectrum of antimicrobial activity against bacteria, yeasts, and fungi. Hence, to confer stability inside a food matrix, such oils can be microencapsulated using whey protein derivatives as the carrier material. For example, WPI was used to encapsulate cardamom essential oil [77]. It was found that the WPI microcapsules obtained had a spherical, regular, and smooth texture and, during storage, it was able to retain the oil at a 30% concentration.
This entry is adapted from the peer-reviewed paper 10.3390/dairy1030016
References
- Yalcin, A. S. Emerging therapeutic potential of whey proteins and peptides. Current pharmaceutical design. 2006, 12(13), 1637-1643.
- Leppala, A. P. Bioactive peptides derived from bovine whey proteins: Opioid and ACEI peptides. Trends in Food Science and Technology. 2001, 11, 347-356.
- Madureira, A. R.; Pereira, C. I.; Gomes, A. M.; Pintado, M. E.; Malcata, F. X. Bovine whey proteins–Overview on their main biological properties. Food Research International. 2007, 40(10), 1197-1211.
- Séverin, S.; Wenshui, X. Milk biologically active components as nutraceuticals. Critical reviews in food science and nutrition. 2005, 45(7-8), 645-656.
- Chou, C. J.; Affolter, M.; Kussmann, M. A nutrigenomics view of protein intake: Macronutrient, bioactive peptides, and protein turnover. In Progress in molecular biology and translational science. 2012, 108, 51-74.
- Bertenshaw, E. J.; Lluch, A.; Yeomans, M. R. Satiating effects of protein but not carbohydrate consumed in a between-meal beverage context. Physiology & Behavior. 2008, 93(3), 427-436.
- Gomes, S. P.; Nyengaard, J. R.; Misawa, R.; Girotti, P. A.; Castelucci, P.; Blazquez, F. H. J.; Ribeiro, A. A. C. Atrophy and neuron loss: Effects of a protein‐deficient diet on sympathetic neurons. Journal of neuroscience research. 2009, 87(16), 3568-3575.
- Bhushan, B.; Jung, Y. C. Wetting, adhesion and friction of superhydrophobic and hydrophilic leaves and fabricated micro/nanopatterned surfaces. Journal of Physics: Condensed Matter. 2008, 20(22), 225010.
- Katayama, M.; Wilson, L. A. Utilization of okara, a byproduct from soymilk production, through the development of soy‐based snack food. Journal of food science. 2008, 73(3), S152-S157.
- Pavlovich-Abril, A.; Rouzaud-Sández, O.; Carvajal-Millán, E.; Navarro, R. E.; Robles-Sánchez, R. M.; Barrón-Hoyos, J. M. Molecular characterization of water extractable arabinoxylans isolated from wheat fine bran and their effect on dough viscosity. LWT. 2016, 74, 484-492.
- Sousa, G. T.; Lira, F. S.; Rosa, J. C.; de Oliveira, E. P.; Oyama, L. M.; Santos, R. V.; Pimentel, G. D. Dietary whey protein lessens several risk factors for metabolic diseases: a review. Lipids in health and disease. 2012, 11(1), 67.
- Alimentarius, C. Milk and milk products. CODEX STAN. 2011, 243-2003.
- Ahn, W. S.; Park, S. J.; Lee, S. Y. Production of poly (3-hydroxybutyrate) from whey by cell recycle fed-batch culture of recombinant Escherichia coli. Biotechnology Letters. 2001, 23(3), 235-240.
- Almeida, G.; Magalhães, R.; Carneiro, L.; Santos, I.; Silva, J.; Ferreira, V.; Teixeira, P. Foci of contamination of Listeria monocytogenes in different cheese processing plants. International journal of food microbiology. 2013, 167(3), 303-309.
- Park, Y. W.; Juárez, M., Ramos, M.; Haenlein, G. F. W. Physico-chemical characteristics of goat and sheep milk. Small ruminant research. 2007, 68(1-2), 88-113.
- Evans, E. W. Uses of milk proteins in formulated foods. Developments in food proteins. 1982.
- Pintado, M. E.; Macedo, A. C.; Malcata, F. X. Technology, chemistry and microbiology of whey cheeses. Food Science and Technology International. 2001, 7(2), 105-116.
- De Wit, J. N. Nutritional and functional characteristics of whey proteins in food products. Journal of dairy science. 1998, 81(3), 597-608.
- Eigel, W. N.; Butler, J. E.; Ernstrom, C. A.; Farrell Jr, H. M.; Harwalkar, V. R.; Jenness, R.; Whitney, R. M. Nomenclature of proteins of cow's milk: fifth revision. Journal of Dairy Science. 1984, 67(8), 1599-1631.
- Brew, K.; Castellino, F. J.; Vanaman, T. C.; & Hill, R. L. The complete amino acid sequence of bovine α-lactalbumin. Journal of Biological Chemistry. 1970, 245(17), 4570-4582.
- Korhonen, H. J. Whey as raw material for development of new products for human nutrition and health: a review. In Milk in nutrition: effects of production and processing factors: proceedings of NJF/NMR-seminar no. 252, Turku, Finland 13.-15.1. NJF-report 102/Edited by: Säde Mantere-Alhonen and Kalle Maijala. Scandinavian Association of Agricultural Scientists. 1995.
- Westhoek and Colleagues.; Available online: http://www.fao.org/fileadmin/user_upload/animalwelfare/Protein_Puzzle_web_1.pdf (accessed on 17 July 2017).
- Lemon, P. W. Do athletes need more dietary protein and amino acids?. International Journal of Sport Nutrition and Exercise Metabolism. 1995, 5(s1), S39-S61.
- Smithers; Geoffrey, W. "Whey and whey proteins—from ‘gutter-to-gold’." International Dairy Journal. 2008, 18.7, 695-704.
- Shang, N.; Chaplot, S.; Wu, J. Food proteins for health and nutrition. In Proteins in Food Processing. 2018, 301-336.
- Suárez, E.; Lobo, A.; Alvarez, S.; Riera, F. A.; Álvarez, R. Demineralization of whey and milk ultrafiltration permeate by means of nanofiltration. Desalination. 2009, 241(1-3), 272-280.
- Wright, B. J.; Zevchak, S. E.; Wright, J. M.; Drake, M. A. The impact of agglomeration and storage on flavor and flavor stability of whey protein concentrate 80% and whey protein isolate. Journal of food science. 2009, 74(1), S17-S29.
- Morifuji, M.; Ishizaka, M.; Baba, S.; Fukuda, K.; Matsumoto, H.; Koga, J.; Higuchi, M. Comparison of different sources and degrees of hydrolysis of dietary protein: effect on plasma amino acids, dipeptides, and insulin responses in human subjects. Journal of Agricultural and Food Chemistry. 2010, 58(15), 8788-8797.
- Buckley, J. D.; Thomson, R. L.; Coates, A. M.; Howe, P. R.; DeNichilo, M. O.; Rowney, M. K. Supplementation with a whey protein hydrolysate enhances recovery of muscle force-generating capacity following eccentric exercise. Journal of Science and Medicine in Sport. 2010, 13(1), 178-181.
- Nongonierma, A. B.; FitzGerald, R. J. The scientific evidence for the role of milk protein-derived bioactive peptides in humans: A Review. Journal of Functional Foods. 2015, 17, 640-656.
- Li-Chan, E. C. Bioactive peptides and protein hydrolysates: research trends and challenges for application as nutraceuticals and functional food ingredients. Current Opinion in Food Science. 2015, 1, 28-37.
- Fekete, A. A.; Givens, D. I.; Lovegrove, J. A. The impact of milk proteins and peptides on blood pressure and vascular function: a review of evidence from human intervention studies. Nutrition research reviews. 2013, 26(2), 177-190.
- Shimizu, M. Food-derived peptides and intestinal functions. Biofactors. 2004, 21(1-4), 43-47.
- Korhonen, H.; Pihlanto-Leppälä, A. Milk-derived bioactive peptides: Formation and prospects for health promotion. Handbook of functional dairy products. 2004, 109-124.
- FitzGerald, R. J.; Meisel, H. Milk protein hydrolysates and bioactive peptides. In Advanced Dairy Chemistry—1 Proteins. 2003, 675-698
- Panchaud, A.; Affolter, M.; Kussmann, M. Mass spectrometry for nutritional peptidomics: how to analyze food bioactives and their health effects. Journal of Proteomics. 2012, 75(12), 3546-3559.
- Layman, D. K.; Lönnerdal, B.; Fernstrom, J. D. Applications for α-lactalbumin in human nutrition. Nutrition reviews. 2018, 76(6), 444-460.
- Ulfman, L. H.; Leusen, J. H.; Savelkoul, H. F.; Warner, J. O.; van Neerven, R. J. Effects of bovine immunoglobulins on immune function, allergy, and infection. Frontiers in nutrition. 2018, 5, 52.
- Walzem, R. L. Health enhancing properties of whey proteins and whey fractions. Blood. 1999, 1, 1-6.
- Hulmi, J. J.; Lockwood, C. M.; Stout, J. R. Effect of protein/essential amino acids and resistance training on skeletal muscle hypertrophy: A case for whey protein. Nutrition & metabolism. 2010, 7(1), 51.
- Chen, W. C.; Huang, W. C.; Chiu, C. C.; Chang, Y. K.; Huang, C. C. Whey protein improves exercise performance and biochemical profiles in trained mice. Medicine and science in sports and exercise, 2014, 46(8), 1517.
- Bell, S. J. Whey protein concentrates with and without immunoglobulins: a review. Journal of medicinal food. 2000, 3(1), 1-13.
- Trachootham, D.; Lu, W.; Ogasawara, M. A.; Valle, N. R. D.; Huang, P. Redox regulation of cell survival. Antioxidants & redox signaling. 2008, 10(8), 1343-1374.
- Neelima; Sharma, R.; Rajput, Y. S.; Mann, B. Chemical and functional properties of glycomacropeptide (GMP) and its role in the detection of cheese whey adulteration in milk: a review. Dairy Science & Technology. 2013, 93, 21–43.
- Markus, C. R.; Olivier, B.; de Haan, E. H. Whey protein rich in α-lactalbumin increases the ratio of plasma tryptophan to the sum of the other large neutral amino acids and improves cognitive performance in stress-vulnerable subjects. The American journal of clinical nutrition. 2002, 75(6), 1051-1056.
- Ganjam, L. S.; Thornton Jr, W. H.; Marshall, R. T.; MacDonald, R. S. Antiproliferative effects of yogurt fractions obtained by membrane dialysis on cultured mammalian intestinal cells. Journal of Dairy Science. 1997, 80(10), 2325-2329.
- Puyol, P.; Perez, M. D.; Ena, J. M.; Calvo, M. Interaction of bovine β-lactoglobulin and other bovine and human whey proteins with retinol and fatty acids. Agricultural and biological chemistry.1991, 55(10), 2515-2520.
- Wu, S. Y.; Pérez, M. D.; Puyol, P.; Sawyer, L. β-Lactoglobulin binds palmitate within its central cavity. Journal of Biological Chemistry. 1999, 274(1), 170-174.
- Wang, Q.; Allen, J. C.; Swaisgood, H. E. Binding of vitamin D and cholesterol to β-lactoglobulin. Journal of Dairy Science. 1997, 80(6), 1054-1059.
- Perez, M. D.; Sanchez, L.; Aranda, P.; Ena, J.; Oria, R.; Calvo, M. Effect of β-lactoglobulin on the activity of pregastric lipase. A possible role for this protein in ruminant milk. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism.1992, 1123(2), 151-155.
- Farrell Jr, H. M.; Behe, M. J.; Enyeart, J. A. Binding of p-nitrophenyl phosphate and other aromatic compounds by β-lactoglobulin. Journal of Dairy Science. 1987, 70(2), 252-258.
- Warme, P. K.; Momany, F. A.; Rumball, S. V.; Tuttle, R. W.; Scheraga, H. A. Computation of structures of homologous proteins alpha-lactalbumin from lysozyme. Biochemistry.1974, 13(4), 768-782.
- Walzem, R. L.; Dillard, C. J.; German, J. B. Whey components: millennia of evolution create functionalities for mammalian nutrition: what we know and what we may be overlooking. Critical reviews in food science and nutrition. 2002, 42(4), 353-375.
- Bosselaers, I. E. M.; Caessens, P. W. J. R.; Van Boekel, M. A. J. S.; Alink, G. M. Differential effects of milk proteins, BSA and soy protein on 4NQO-or MNNG-induced SCEs in V79 cells. Food and chemical toxicology. 1994, 32(10), 905-909.
- Laursen, I.; Briand, P.; Lykkesfeldt, A. E. Serum albumin as a modulator on growth of the human breast cancer cell line, MCF-7. Anticancer Research. 1990, 10(2A), 343.
- Mitra, A. K.; Mahalanabis, D.; Ashraf, H.; Unicomb, L.; Eeckels, R.; Tzipori, S. Hyperimmune cow colostrum reduces diarrhoea due to rotavirus: a double‐blind, controlled clinical trial. Acta paediatrica. 1995, 84(9), 996-1001.
- Loimaranta, V.; Laine, M.; Soèderling, E.; Vasara, E.; Rokka, S.; Marnila, P.; Tenovuo, J. Effects of bovine immune and non‐immune whey preparations on the composition and pH response of human dental plaque. European journal of oral sciences. 1999, 107(4), 244-250.
- Freedman, D. J.; Tacket, C. O.; Delehanty, A.; Maneval, D. R.; Nataro, J.; Crabb, J. H. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. The Journal of infectious diseases. 1998, 177(3), 662-667.
- Okhuysen, P. C.; Chappell, C. L.; Crabb, J.; Valdez, L. M.; Douglass, E. T.; DuPont, H. L. Prophylactic effect of bovine anti-Cryptosporidium hyperimmune colostrum immunoglobulin in healthy volunteers challenged with Cryptosporidium parvum. Clinical Infectious Diseases. 1998, 26(6), 1324-1329.
- Sharpe, S. J.; Gamble, G. D.; Sharpe, D. N. Cholesterol-lowering and blood pressure effects of immune milk. The American journal of clinical nutrition. 1994, 59(4), 929-934.
- Kinsella, J. E.; Whitehead, D. M. Proteins in whey: chemical, physical, and functional properties. In Advances in food and nutrition research. 1989, 33, 343-438.
- Krzeminski, A.; Prell, K. A.; Busch-Stockfisch, M. Whey protein–pectin complexes as new texturising elements in fat-reduced yoghurt systems. Int Dairy J. 2014, 36, 118–127.
- Kuhn, K. R.; Cunha, R. L. Flaxseed oil–whey protein isolate emulsions: effect of high pressure homogenization. Journal of Food Engineering. 2012, 111(2), 449-457.
- Akalın, A. S.; Unal, G.; Dinkci, N.; Hayaloglu, A. A. Microstructural, textural, and sensory characteristics of probiotic yogurts fortified with sodium calcium caseinate or whey protein concentrate. Journal of Dairy Science. 2012, 95(7), 3617-3628.
- Nadeem, M.; Muhammad Anjum, F.; Murtaza, M. A.; Mueen-ud-Din, G. Development, characterization, and optimization of protein level in date bars using response surface methodology. The Scientific World Journal, 2012.
- Leksrisompong, P.; Gerard, P.; Lopetcharat, K.; Drake, M. Bitter taste inhibiting agents for whey protein hydrolysate and whey protein hydrolysate beverages. Journal of food science. 2012, 77(8), S282-S287.
- Liu, J.; Wang, X.; Zhao, Z. Effect of whey protein hydrolysates with different molecular weight on fatigue induced by swimming exercise in mice. Journal of the Science of Food and Agriculture. 2014, 94(1), 126-130.
- Gad, A. S.; Khadrawy, Y. A.; El-Nekeety, A. A.; Mohamed, S. R.; Hassan, N. S.; Abdel-Wahhab, M. A. Antioxidant activity and hepatoprotective effects of whey protein and Spirulina in rats. Nutrition. 2011, 27(5), 582-589.
- Nedovic, V. A.; Obradovic, B.; Leskosek-Cukalovic, I.; Vunjak-Novakovic, G. Immobilized yeast bioreactor systems for brewing—recent achievements. In Engineering and manufacturing for biotechnology. 2001, 277-292.
- de Vos, P.; Faas, M. M.; Spasojevic, M.; Sikkema, J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. International dairy journal. 2010, 20(4), 292-302.
- Desai, K. G. H.; Jin Park, H. Recent developments in microencapsulation of food ingredients. Drying technology. 2005, 23(7), 1361-1394
- Wandrey, C.; Bartkowiak, A.; Harding, S. E. Materials for encapsulation. In Encapsulation technologies for active food ingredients and food processing. 2010, 31-100.
- Martin, A. H.; De Jong, G. A. H. Enhancing the in vitro Fe 2+ bio-accessibility using ascorbate and cold-set whey protein gel particles. Dairy science & technology. 2012, 92(2), 133-149.
- Pérez-Masiá, R.; López-Nicolás, R.; Periago, M. J.; Ros, G., Lagaron, J. M.; López-Rubio, A. Encapsulation of folic acid in food hydrocolloids through nanospray drying and electrospraying for nutraceutical applications. Food Chemistry. 2015, 168, 124-133.
- Gülseren, İ.; Fang, Y.; Corredig, M. Complexation of high methoxyl pectin with ethanol desolvated whey protein nanoparticles: physico-chemical properties and encapsulation behaviour. Food & function. 2012, 3(8), 859-866.
- Parris, N.; Cooke, P. H.; Hicks, K. B. Encapsulation of essential oils in zein nanospherical particles. Journal of agricultural and food chemistry. 2005, 53(12), 4788-4792.
- Mehyar, G. F.; Al‐Isamil, K. M.; Al‐Ghizzawi, H. A. M.; Holley, R. A. Stability of cardamom (Elettaria Cardamomum) essential oil in microcapsules made of whey protein isolate, guar gum, and carrageenan. Journal of food science. 2014, 79(10), C1939-C1949.
