Heavy metals and metalloids (HMs) are environmental pollutants, most notably cadmium, lead, arsenic, mercury, and chromium. When HMs accumulate to toxic levels in agricultural soils, these non-biodegradable elements adversely affect crop health and productivity. The toxicity of HMs on crops depends upon factors including crop type, growth condition, and developmental stage; nature of toxicity of the specific elements involved; soil physical and chemical properties; occurrence and bioavailability of HM ions in the soil solution; and soil rhizosphere chemistry. HMs can disrupt the normal structure and function of cellular components and impede various metabolic and developmental processes.
- heavy metals
- arable lands
- agricultural practices
1. Introduction
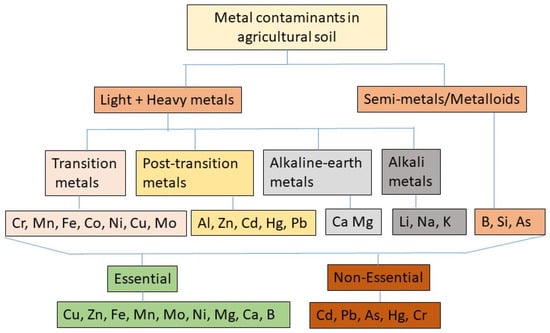
2. Sources of HM Contamination in Arable Lands
2.1. Application of Chemical Fertilizers
| Fertilizers | Cadmium Content (mg Kg−1) | |
|---|---|---|
| Based on Product | Based on P Content | |
| Complete fertilizer | 23–29 | 418–527 |
| Single superphosphate | 16–26 | 186–302 |
| Superphosphate | 13–34 | 151–395 |
| Rock phosphate | 7.2–47 | 54–303 |
| High analysis fertilizer | <0.6–5.6 | 15–118 |
| Double superphosphate | <0.6–12 | <3.6–72 |
| Triple superphosphate | 0.8–7.0 | 24–35 |
| Mono-ammonium phosphate | 1.8–8.1 | 12–37 |
| Di-ammonium phosphate | 4.3–6.6 | 22–28 |
| Elements | MAX | MIN | Fold Difference |
|---|---|---|---|
| mg Kg−1 Soil | |||
| Cd | 0.65 | 0.06 | 10.8 |
| Cu | 171.5 | 21.0 | 8.2 |
| Ni | 36.9 | 28.7 | 1.3 |
| Pb | 38.0 | 20.5 | 1.9 |
| Zn | 433 | 71.9 | 6 |
2.2. Pesticide Application
| Chemical Name | Formula | HM Elements |
|---|---|---|
| Insecticides | ||
| Aluminum phosphide | AIP | Al |
| Aluminum silicate | Al2Si2O7 | Al |
| Arsenic acid | H3AsO4 | As |
| Copper acetoarsenite | C4H6As6Cu4O16 | As, Cu |
| Copper oxide | Cu2O | Cu |
| Copper carbonate | CH2Cu2O | Cu |
| Copper naphthenate | C22H14CuO4 | Cu |
| Lead arsenate | AsHO4Pb | As, Pb |
| Lithium perfluorooctane sulfonate | C8F17LiO3S | Li |
| Sodium meta-arsenite | NaAsO2 | As |
| Fungicide | ||
| Copper oxide | CuO | Cu |
| Copper bis(3-phenylsalicylate) | C26H18CuO6 | Cu |
| Copper abietate | C40H58CuO4 | Cu |
| Copper acetate | Cu2(CH3COO)4 | Cu |
| Copper carbonate | CH2Cu2O | Cu |
| Copper chloride | CuCl2 | Cu |
| Copper hydroxide | H2O2Cu | Cu |
| Copper naphthenate | C22H14CuO4 | Cu |
| Copper oxychloride | (ClCu2H3O3)2 | Cu |
| Copper sulphate | CuSO4-Ca(OH)2 | Cu |
| Mercuric oxide | HgO | Hg |
| Mercurous chloride | Hg2Cl2 | Hg |
| Methoxyethylmercury chloride | C3H7ClHgO | Hg |
| Methoxyethylmercury acetate | C5H10HgO3 | Hg |
| Phenyl mercuric acetate | C8H8O2Hg | Hg |
| Phenylmercury chloride | C6H5ClHg | Hg |
| Phenylmercury nitrate | C6H5HgNO3 | Hg |
| Sodium arsenite | NaAsO2 | As |
| Zinc borate | ZnB3O4(OH)3 | Zn, B |
| Zinc oxide | ZnO | Zn |
| Zineb | C4H6N2S4Zn | Zn |
| Herbicides | ||
| Arsenic acid | H3AsO4 | As |
| Calcium arsenate | As2Ca3O8 | As |
| Sodium arsenite | NaAsO2 | As |
| Cacodylic acid | (CH3)2AsO(OH) | As |
| Rodenticides | ||
| Barium carbonate | BaCO3 | Ba |
| Zinc phosphide | Zn3P2 | Zn |
| Thallium sulfate | Tl2SO4 | Tl |
| Defoliants | ||
| Sodium dichromate | Na2Cr2O7 | Cr |
| Zinc chloride | ZnCl2 | Zn |
| Mercuric chloride | HgCl2 | Hg |
| Trade Name | Technical Name | Metal Impurities (ppb) * |
|---|---|---|
| Insecticides | Defarge et al. [38] | |
| Pyrinex® | Chlorpyriphos | As (390), Cr (800), Ni (1200) |
| Polysect® | Acetamiprid | Ni (50) |
| Fungicides | Defarge et al., [38] | |
| Eyetak® | Prochloraz | As (200), Co (90), Cr (200), Ni (190), Pb (12) |
| Folpan® | Folpet | As (260), Cr (2000), Ni (1200) |
| Maronee® | Tebuconazole | As (90), Co (50), Cr (100) |
| Opus® | Epoxiconazole | Cr (90), Ni (60) |
| Pictor® | Boscalid | As (300), Co (275), Cr (1000), Ni (600) |
| Teldor® | Fenhexamid | As (575), Cr (800), Ni (800) |
| Herbicides | Defarge et al. [38] | |
| R 3+® | Glyphosate-based formulations | As (375), Co (50), Cr (175), Ni (20) |
| R Bioforce® | As (260), Cr (200), Ni (120) | |
| R Express® | As (60) | |
| R GT+® | As (450), Co (150), Cr (100), Ni (50), Pb (10) | |
| R WeatherMax® | As (500), Cr (100), Ni (20), Pb (10) | |
| Bayer GC® | As (75), Co (60), Cr, (110) Ni (20) | |
| Clinic EV® | As (400), Co (90), Cr (150), Ni (20) | |
| Glyfos® | As (200), Cr (1100), Ni (50), Pb (30) | |
| Glyphogan® | As (320), Co (125), Cr (100), Ni (40) | |
| Pavaprop-G® | Cr (110), N (190) | |
| Radical Tech+® | As (270), Co (70), Cr (50), Ni (50) | |
| Lonpar® | 2,4-D | As (160), Cr (150), Ni (180) |
| Matin® | Isoproturon | As (100), Cr (100), Ni (30), Pb (25) |
| Starane® | Fluoroxypyr | As (75), Cr (250), Ni (100), Pb (100) |
| Insecticides | Alnuwaiser [39] | |
| Sniper® | Fipronil | Zn (506), Cu (423), Cr (746), Co (275), Pb (88) |
| CyperCel® | Cypermethrin | Zn (2389), Cu (669), Cr (373), Co (18), Pb (807) |
| CyperSafe® | Cypermethrin | Zn (968), Cu (464), Cr (10), Co (6), Pb (119) |
| Scope 60® | Asaybrmthrin | Zn (527), Cu (539), Cr (437), Co (23), Pb (39) |
| Brodor® | Permethrin | Zn (10), Cr (16), Pb (186) |
| Clash® | Acephate + Buprofezin | Zn (1078), Cr (73), Co (39), Pb (1316) |
| Acefed® | Mithomail | Cu (19), Cr (48), Co (4), Pb (121) |
| Lanid® | - | Cu (128), Cr (60), Pb (98) |
| Probalt® | - | Cu (179), Cr (85), Co (25), Pb (46) |
| Nourcam® | - | - |
| Madar® | - | Zn (10), Cu (66), Cr (16), Co (10), |
| PifPaf® | - | Cu (110), Co (5), Thallium, Tl (19), Pb (12) |
| Paygon® | - | Zn (52), Tl (15), Pb (19) |
2.3. Application of Livestock Manures and Compost
| Source | Level | Zn | Cu | Pb | Cd | Cr | Hg | As | Ni |
|---|---|---|---|---|---|---|---|---|---|
| Pig | MAX | 4639 | 1288 | 23 | 60 | 85 | 0.3 | 89 | 19 |
| MIN | 100 | 73 | 0.3 | 0.04 | 3.5 | 0.0 | 0.01 | 4.7 | |
| FD | 46 | 18 | 77 | 1500 | 24 | - | 8900 | 4.0 | |
| Chicken | MAX | 578 | 314 | 33 | 4.1 | 251 | 0.5 | 23 | 39 |
| MIN | 166 | 18 | 3.0 | 0.03 | 4.0 | 0.02 | 0.05 | 5.2 | |
| FD | 3.5 | 17 | 11 | 137 | 63 | 25 | 460 | 7.5 | |
| Duck | MAX | 682 | 199 | 41 | 2.5 | 64 | 0.07 | 6.8 | 16 |
| MIN | 98 | 35 | 4.5 | 0.3 | 7.0 | 0.03 | 0.01 | 8.4 | |
| FD | 7.0 | 5.7 | 9.1 | 8.3 | 9.1 | 2.3 | 680 | 1.9 | |
| Poultry | MAX | 682 | 314 | 41 | 4.1 | 251 | 0.5 | 23 | 39 |
| MIN | 77 | 15 | 2.0 | 0.03 | 2.5 | 0.02 | 0.01 | 5.2 | |
| FD | 8.9 | 21 | 21 | 137 | 100 | 25 | 2300 | 7.5 | |
| Cattle | MAX | 816 | 174 | 32 | 3.4 | 79 | 0.6 | 6.3 | 19 |
| MIN | 49 | 12 | 1.6 | 0.04 | 0.8 | 0.02 | 0.01 | 4.2 | |
| FD | 17 | 15 | 20 | 85 | 99 | 30 | 630 | 4.5 | |
| Sheep | MAX | 431 | 215 | 20 | 1.4 | 22 | 2.4 | 2.6 | 12 |
| MIN | 42 | 8.4 | 1.7 | 0.3 | 8.0 | 0.2 | 0.6 | 1.2 | |
| FD | 10 | 26 | 12 | 4.7 | 2.8 | 12 | 4.3 | 10 |
2.4. Application of Sewage-Sludge-Based Biosolids
| Elements | Maximum Permissible Level (mg Kg−1) | Cumulative Loading Rate (Kg ha−1) | Monthly Average Concentrations (mg Kg−1) | Annual Loading Rate (Kg ha−1) |
|---|---|---|---|---|
| As | 75 | 41 | 41 | 2.0 |
| Cd | 85 | 39 | 39 | 1.9 |
| Cr | 3000 | 3000 | 1200 | 150 |
| Cu | 4300 | 1500 | 1500 | 75 |
| Pb | 840 | 300 | 300 | 15 |
| Hg | 57 | 17 | 17 | 0.9 |
| Ni | 420 | 420 | 420 | 21 |
| Se | 100 | 100 | 36 | 5.0 |
| Zn | 7500 | 2800 | 2800 | 140 |
2.5. Land Irrigation
| Elements | Cr | Cu | Zn | As | Cd | Pb | Ni | References |
|---|---|---|---|---|---|---|---|---|
| mg L−1 | ||||||||
| Max level (FD) * |
2.13 (21.3) |
4.62 (23.1) |
15.20 (7.6) |
0.52 (5.2) |
0.02 (2.0) |
1.15 (0.2) |
- | Ahmed et al. [16] |
| Max level (FD) |
0.94 (9.4) |
0.61 (3.1) |
0.86 (0.4) |
- | 0.04 (4.0) |
0.19 (0.04) |
0.12 (0.6) |
Berihun et al. [58] |
| FAO limit | 0.10 | 0.20 | 2.00 | 0.10 | 0.01 | 5.00 | 0.20 | |
This entry is adapted from the peer-reviewed paper 10.3390/agronomy13061521
