Asthma is a heterogeneous disease characterized by chronic airway inflammation. Group 2 innate lymphoid cells (ILC2) play an important role in the pathogenesis of asthma. ILC2s lack antigen-specific receptors and respond to epithelial-derived cytokines, leading to the induction of airway eosinophilic inflammation in an antigen-independent manner. Additionally, ILC2s might be involved in the mechanism of steroid resistance. Numerous studies in both mice and humans have shown that ILC2s induce airway inflammation through inflammatory signals, including cytokines and other mediators derived from immune or non-immune cells.
1. Introduction
Asthma is a heterogeneous disease, characterized by chronic airway inflammation, variable expiratory airflow limitation, and a history of respiratory symptoms, such as wheezing, shortness of breath, chest tightness, and cough, which vary in intensity and over time [
1]. It affects approximately 300 million people worldwide, with the prevalence increasing in developed countries.
Recent clinical and translational research has demonstrated that asthma is a heterogeneous disease comprising various phenotypes and endotypes. In terms of phenotype, asthma encompasses eosinophilic asthma, neutrophilic asthma, mixed granulocytic asthma, and pauci-granulocytic asthma [
2]. Mixed granulocytic asthma is a phenotype characterized by increased levels of both eosinophils and neutrophils. Pauci-granulocytic asthma is a phenotype characterized by normal levels of both eosinophils and neutrophils [
3]. Among these phenotypes of asthma, eosinophilic asthma, characterized by eosinophilia in the airways or blood driven by type 2 immune responses, can be induced by allergic and non-allergic mechanisms, mainly involving T helper type 2 (Th2) cells and group 2 innate lymphoid cells (ILC2), respectively.
Over the past decade, ILCs have been identified as a component of the innate immune system that can interact with various hematopoietic and non-hematopoietic cells to coordinate immunity, inflammation, and homeostasis in multiple organs throughout the body [
4]. Unlike T cells and B cells, ILCs lack antigen-specific receptors and lineage (Lin) markers, and they cause antigen-non-specific immune responses [
5,
6]. ILCs consist of three subsets—ILC1s, ILC2s, and ILC3s—which are characterized by their transcription factors and the cytokines that they produce. These subsets correspond to Th1, Th2, and Th17 cells, respectively. Among these ILCs, ILC2s have been discovered in the gut, spleen, liver, and bone marrow [
5,
7,
8]. ILC2s produce a large amount of IL-5 and IL-13 in response to epithelial-cell-derived cytokines, such as IL-25, IL-33, and thymic stromal lymphopoietin (TSLP), which are considered to play an essential role in the pathogenesis of allergic disorders, including asthma.
Currently, to achieve asthma control and to reduce the risk of exacerbations, various types of biologics have been used. Among biologics, dupilumab and tezepelumab target IL-4Rα and TSLP, respectively, and IL-4 and TSLP acted on ILC2s directly in
in vivo or
in vitro studies; therefore, these biologics might attenuate ILC2-mediated asthma. In a study using sorted peripheral blood ILC2s
in vitro, the expression of type 2 cytokine mRNA was shown to be significantly decreased in asthmatics treated with dupilumab [
119].
2. Airway Inflammation in Asthma Pathogenesis
The characteristics of asthma are chronic airway inflammation and airway hyperresponsiveness (AHR). Airway inflammation involves various inflammatory cells, such as eosinophils, neutrophils, lymphocytes, and mast cells, as well as airway structural cells including airway epithelial cells, fibroblasts, and airway smooth muscle cells, along with various humoral factors [
9]. Persistent airway inflammation causes airway remodeling, which leads to irreversible airflow limitations. Furthermore, AHR is considered to be primarily induced by airway inflammation, whereas AHR is induced even when airway inflammation is mild with airway remodeling, because repeated bronchoconstriction promotes airway remodeling [
10].
The airways of asthmatic patients exhibit pathologies, such as goblet cell metaplasia, excessive subepithelial collagen deposition, airway smooth muscle hyperplasia, and increased vascularity [
11]. These findings are considered to be caused by inflammatory mediators, including cytokines and chemokines, produced by inflammatory cells and airway structural cells (
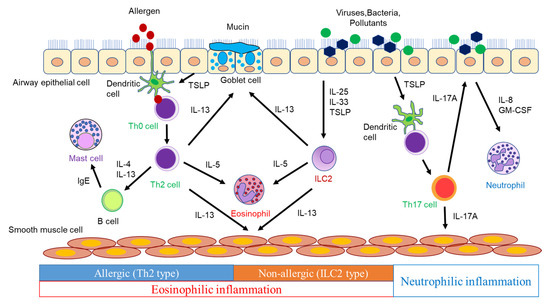
Figure 1).
Figure 1. The pathogenesis of airway inflammation in asthma.
Dendritic cells (DCs) phagocytose antigens and present them to naïve CD4 T cells, which differentiate into Th2 cells via the surrounding cytokine and produce type 2 cytokines, such as IL-4, IL-5, and IL-13. IL-4 induces the expression of vascular cell adhesion molecule-1 (VCAM-1) in vascular endothelial cells, which leads to eosinophil aggregation. IL-4 also induces the isotype class switching of B-cells to IgE synthesis [
12]. Antigen-specific IgE produced by B cells activates mast cells, which release inflammatory mediators such as histamine and leukotrienes (LTs), triggering an immediate asthmatic response [
12]. IL-4 plays a pivotal role in Th2 cell differentiation. IL-13, which shares IL-4 receptor α (IL-4Rα), has many functions that overlap with those of IL-4 [
13]. IL-13 induces airway smooth muscle contraction, mucus hypersecretion, and inducible nitric oxide synthase (iNOS) in bronchial epithelial cells, resulting in an increased level of fractional exhaled nitric oxide (FeNO), in addition to the IL-4-mediated production of IgE and the increased expression of VCAM-1 in vascular endothelial cells [
13,
14]. IL-5 promotes the proliferation, maturation, activation, and survival of eosinophils and the translocation of eosinophils from the bone marrow into the systemic circulation [
13,
15]. These cytokines play a crucial role in the induction of allergic airway inflammation and the pathogenesis of asthma. Moreover, naïve CD4 T cells differentiate into Th17 cells in the presence of IL-6, IL-21, and TGF-β [
16]. Th17 cells produce IL-17A, IL-17F, and IL-22 and contribute to the defense against extracellular parasites and autoimmune diseases. However, their involvement in asthma has also been demonstrated. In a murine model of asthma, the adoptive transfer of antigen-specific Th17 cells mediated airway neutrophilic inflammation and AHR, which promoted steroid resistance [
17]. Thus, IL-17 is involved in severe asthma, neutrophilic asthma, asthma exacerbations, and airway remodeling involving the recruitment of neutrophils [
18]. However, anti-IL-17 biologics provided no clinical benefit in asthmatics [
19]. Therefore, it is necessary to design clinical trials with neutrophilic asthmatics.
++ILC2s provide host defense against helminth infection, contributing to inflammatory responses and tissue repair. ILC2s depend on transcription factor GATA3 for their development and function, and they produce significant amounts of IL-5 and IL-13 in response to IL-25, IL-33, and TSLP, leading to type 2 immune responses. In addition, since ILC2s induce type 2 immune responses in Rag1
−/− mice lacking T cells and B cells, ILC2s have been shown to produce type 2 cytokines in a T-cell-independent manner [
20]. Thus, ILC2s play an essential role in innate-immunity-mediated type 2 airway inflammation. ILC3s require transcription factor RORγ for their induction and produce IL-17A, IL-17F, and IL-22 [
21]. The ILC3-mediated production of IL-17, as well as Th17 cells, may contribute to asthma, especially neutrophilic asthma.
This entry is adapted from the peer-reviewed paper 10.3390/biom13060893