Medicine waste is a global issue, with economic, environmental, and social consequences that are only predicted to worsen. To address a lack of technological advancements to enable medicine reuse a Smart Packaging and Returns Assessment System (SPaRAS) was proposed to validate the storage conditions and streamline the assessment of returned medicines. The Smart Packaging System (SPS) will record the storage conditions of medication while in patient care. The companion Returns Assessment System (RAS) will efficiently communicate with the SPS through RFID, configure the sensors within the SPS to the needs of its assigned medicine and assess the returns against tailored eligibility criteria. The increased safety and efficiency provided by SPaRAS addresses the concerns of large pharmaceutical companies and the public, offering a method to reuse previously owned medication and reduce the effects of unnecessary medicine waste.
- SPaRAS
- Medicine Reuse
- CPSC
- Circular Pharmaceutical Supply Chain
- Circular Economy
- Environmental Sensors
- Smart Packaging
1. Background
| Smarter product use, design, and manufacturing | |
| R0. Refuse | Minimise levels of raw materials by making products redundant. |
| R1. Rethink | Alter product design to maximise its use. |
| R2. Reduce | Consume fewer raw materials by promoting efficient manufacturing. |
| Extend lifespan of products and components | |
| R3. Reuse | Second-hand use of products that retain functionality. |
| R4. Repair | Increase the longevity of a product through maintenance. |
| R5. Refurbishment | Restore functionality to an older product. |
| R6. Remanufacture | Create a new product with the same function using components from an unusable product. |
| R7. Repurpose | Create a new product with a different function using components from an unusable product. |
| Useful application of materials | |
| R8. Recycle | Process and reusing waste materials. |
| R9. Recover | Recover energy from waste materials. |
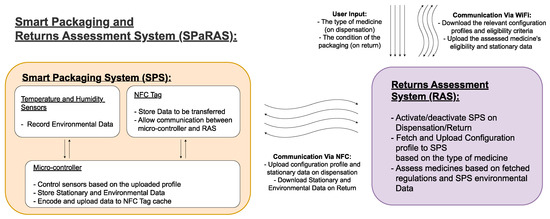
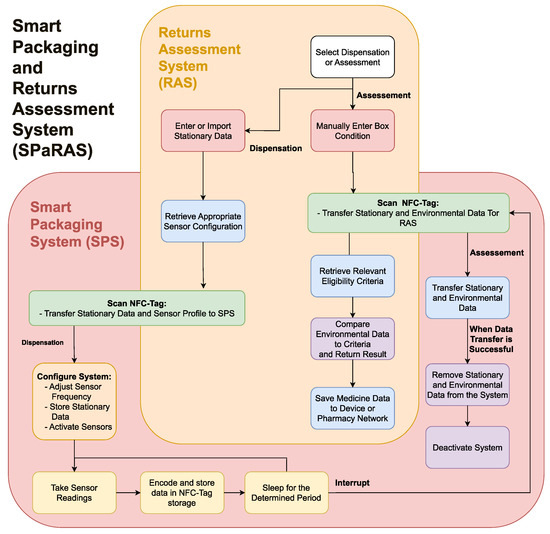
2. A Smart Packaging and Returns Assessment System (SPaRAS)
| Criteria | Relevance |
|---|---|
| System Basis: | |
| Ensure the safety of redispensed medicines. | Addresses public and stakeholder concerns over medicine reuse being dangerous. |
| Increase the efficiency of assessing returned medicines. | Addresses stakeholder concerns of medicine reuse being too expensive. |
| System guidelines: | |
| Use cheap/reusable components. | Influences companies by reducing costs. |
| Influences the public by not drastically increasing the cost of medicine and by the morality of recycling components. | |
| Ensures the system is usable by those with disabilities. | Allows the whole population to accept the new system. |
| Minimises changes to the exterior and functionality of medicine boxes. | Reduces the chance of public aversion to change. |
-
Work reliably for the patient-owned duration;
-
Efficiently communicate with the assessment system;
-
Fit within a medicine box.The RAS will need to:
-
Efficiently transfer data with the smart packaging system;
-
Control functionality of the SPS;
-
Accurately compare data from the SPS to determine eligibility;
-
Be easy to use without extensive training.
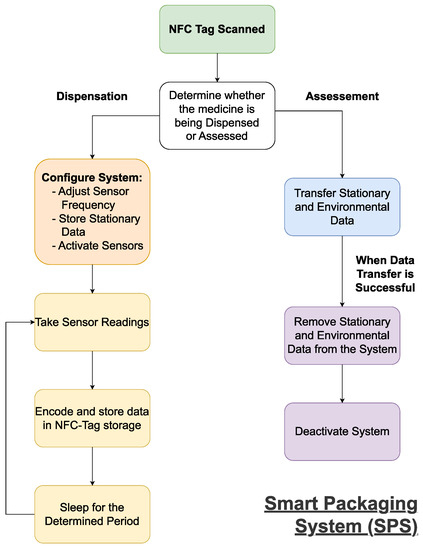
2.1. A Streamlined Smart Pharmaceutical Packaging System
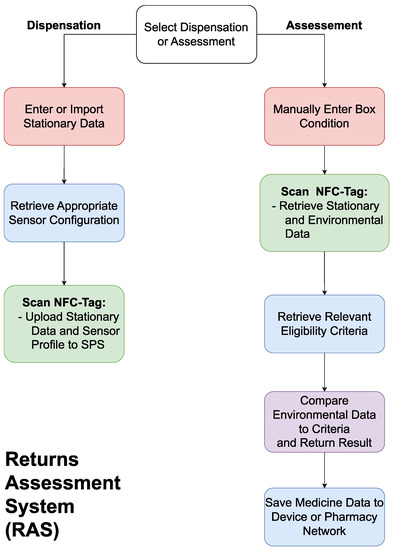
2.2. A Returns Assessment System
2.3. Potential Applications and Future Developments
This entry is adapted from the peer-reviewed paper 10.3390/technologies11030075
References
- Alhamad, H.; Patel, N.; Donyai, P. Towards Medicines Reuse: A Narrative Review of the Different Therapeutic Classes and Dosage Forms of Medication Waste in Different Countries. Pharmacy 2020, 8, 230.
- Freitas, L.; Radis-Baptista, G. Pharmaceutical Pollution and Disposal of Expired, Unused, and Unwanted Medicines in the Brazilian Context. J. Xenobiot. 2021, 11, 61–76.
- González-Alonso, S.; Merino, L.; Esteban, S.; López de Alda, M.; Barceló, D.; Durán, J.; López-Martínez, J.; Aceña, J.; Pérez, S.; Mastroianni, N.; et al. Occurrence of pharmaceutical, recreational and psychotropic drug residues in surface water on the northern Antarctic Peninsula region. Environ. Pollut. 2017, 229, 241–254.
- Depledge, M. Reduce drug waste in the Environment. Nature 2011, 478, 36.
- Evaluation of the Scale, Causes and Costs of Waste Medicines. Available online: https://discovery.ucl.ac.uk/id/eprint/1350234/1/Evaluation_of_NHS_Medicines_Waste__web_publication_version.pdf (accessed on 14 December 2022).
- Mendes, Z.; Crisostomo, S.; Martins, A.; Batel Marques, F.; Rodrigues, V.; Fontes Ribeiro, C. PHP13 The Cost of Wasted Medicines in Portugal. Value Health 2007, 10, A364.
- Vogler, S.; de Rooij, R. Medication wasted—Contents and costs of medicines ending up in household garbage. Res. Social Adm. Pharm. 2018, 14, 1140–1146.
- Almanie, S.; Holdford, D. Economic impact of waste in Prescribing, Dispensing, and Medication consumption in the United States. Value Health 2015, 18, A81–A82.
- Poll: Nearly 1 in 4 Americans Taking Prescription Drugs Say It’s Difficult to Afford Their Medicines, including Larger Shares among Those with Health Issues, with Low Incomes and Nearing Medicare Age. Available online: https://www.kff.org/health-costs/press-release/poll-nearly-1-in-4-americans-taking-prescription-drugs-say-its-difficult-to-afford-medicines-including-larger-shares-with-low-incomes/ (accessed on 20 February 2022).
- Health-Care Waste. Available online: https://www.who.int/news-room/fact-sheets/detail/health-care-waste (accessed on 21 March 2022).
- White, D.; Lapworth, D.; Civil, W.; Williams, P. Tracking changes in the occurrence and source of pharmaceuticals within the River Thames, UK; from source to sea. Environ. Pollut. 2019, 249, 257–266.
- Szymonik, A.; Lach, J.; Malińska, K. Fate and Removal of Pharmaceuticals and Illegal Drugs Present in Drinking Water and Wastewater. Ecol. Chem. Eng. 2017, 24, 65–85.
- Rabiet, M.; Togola, A.; Brissaud, F.; Seidel, J.; Budzinski, H.; Elbaz-Poulichet, F. Consequences of Treated Water Recycling as Regards Pharmaceuticals and Drugs in Surface and Ground Waters of a Medium-sized Mediterranean Catchment. Environ. Sci. Technol. 2006, 40, 5282–5288.
- Letsinger, S.; Kay, P.; Rodríguez-Mozaz, S.; Villagrassa, M.; Barceló, D.; Rotchell, J. Spatial and temporal occurrence of pharmaceuticals in UK estuaries. Sci. Total Environ. 2019, 678, 74–84.
- Lutterbeck, C.; Kern, D.; Machado, Ê.; Kümmerer, K. Evaluation of the toxic effects of four anti-cancer drugs in plant bioassays and its potency for screening in the context of waste water reuse for irrigation. Chemosphere 2015, 135, 403–410.
- Pires, A.; Almeida, Â.; Calisto, V.; Schneider, R.; Esteves, V.; Wrona, F.; Soares, A.; Figueira, E.; Freitas, R. Hediste diversicolor as bioindicator of pharmaceutical pollution: Results from single and combined exposure to carbamazepine and caffeine. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016, 188, 30–38.
- Brozinski, J.; Lahti, M.; Meierjohann, A.; Oikari, A.; Kronberg, L. The Anti-Inflammatory Drugs Diclofenac, Naproxen and Ibuprofen are found in the Bile of Wild Fish Caught Downstream of a Wastewater Treatment Plant. Environ. Sci. Technol. 2013, 47, 342–348.
- Gilbert, N. Drug waste harms fish. Nature 2011, 476, 265.
- Kovacs, E.; Silaghi-Dumitrescu, L.; Kovacs, M.; Roman, C. Determination of the Uptake of Ibuprofen, Ketoprofen, and Diclofenac by Tomatoes, Radishes, and Lettuce by Gas Chromatography–Mass Spectrometry (GC–MS). Anal. Lett. 2021, 54, 314–330.
- Litchman, M.; Oser, T.; Wawrzynski, S.; Walker, H.; Oser, S. The Underground Exchange of Diabetes Medications and Supplies: Donating, Trading, and Borrowing, Oh My! J. Diabetes Sci. Technol. 2020, 14, 1000–1009.
- Novak, S.; Håkansson, A.; Martinez-Raga, J.; Reimer, J.; Krotki, K.; Varughese, S. Nonmedical use of prescription drugs in the European Union. BMC Psychiatry 2016, 16, 274.
- Lipari, R.; Williams, M.; van Horn, S. Why do adults misuse prescription drugs? In The CBHSQ Report, 1st ed.; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK458284/ (accessed on 24 April 2022).
- Deaths Related to Drug Poisoning in England and Wales: 2020 Registrations. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsrelatedtodrugpoisoninginenglandandwales/2020 (accessed on 24 April 2022).
- Khandelwal, N.; Duncan, I.; Ahmed, T.; Rubinstein, E.; Pegus, C. Oral Chemotherapy Program Improves Adherence and Reduces Medication Wastage and Hospital Admissions. J. Natl. Compr. Canc. Netw. 2012, 10, 618–625.
- Vats, V.; Ziang, J.; Lee, K.; Schumock, G.; Khandelwal, N. PIH29 Comparison of Adherence, Persistence and Medication Wastage in 30-Day Versus 90-Day Refill Channels. Value Health 2009, 12, A167.
- Lai, P.; Tan, K.; Lee, H.; Wong, Y.; Azhari Wasi, N.; Sim, S. Effectiveness of an intervention to increase the knowledge, attitude, and practice regarding the return and disposal of unused medications. Malays. Fam. Physician 2021, 16, 56–63.
- Afanasjeva, J.; Gruenberg, K. Pharmacists as environmental stewards: Strategies for minimizing and managing drug waste. Sustain. Chem. Pharm. 2019, 13, 100164.
- State Prescription Drug Repository Programs. Available online: https://www.ncsl.org/research/health/state-prescription-drug-return-reuse-and-recycling.aspx (accessed on 28 April 2022).
- Drug Donation Repository. Available online: https://safenetrx.org/drug-donation-repository (accessed on 28 April 2022).
- Wyoming Department of Health. Available online: https://health.wyo.gov/healthcarefin/medicationdonation/donate-meds/ (accessed on 28 April 2022).
- Drug Recycling Program. Available online: https://www.tcmsok.org/drug-recycling-program (accessed on 28 April 2022).
- Sirum. Available online: https://sirum.org/ (accessed on 28 April 2022).
- Givmed. Available online: https://givmed.org/en/ (accessed on 28 April 2022).
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2017, 127, 221–232.
- Ravindran, A.; Scsavnicki, S.; Nelson, W.; Gorecki, P.; Franz, J.; Oberloier, S.; Meyer, T.; Barnard, A.; Pearce, J. Open Source Waste Plastic Granulator. Technologies 2019, 7, 74.
- National Agreement on the Circular Economy. Available online: https://www.government.nl/topics/circular-economy/documents/discussion-documents/2017/01/24/national-agreement-on-the-circular-economy (accessed on 21 September 2022).
- Close the Loop. Available online: https://www.close-the-loop.be/en/case/285/vigga (accessed on 20 February 2023).
- Carter, C.; Ellram, L. Reverse logistics: A review of the literature and framework for future investigation. J. Bus. Logist. 1998, 19, 85–102. Available online: https://www.proquest.com/scholarly-journals/reverse-logistics-review-literature-framework/docview/212642143/se-2 (accessed on 15 September 2022).
- Hrouga, M.; Sbihi, A.; Chavallard, M. The potentials of combining Blockchain technology and Internet of Things for digital reverse supply chain: A case study. J. Clean. Prod. 2022, 337, 130609.
- Gayialis, S.P.; Kechagias, E.P.; Papadopoulos, G.A.; Panayiotou, N.A. A Business Process Reference Model for the Development of a Wine Traceability System. Sustainability 2022, 14, 11687.
- Sadowski, A. Reverse Logistics for Sustainable Waste-Management Processes. In Sustainable Logistics; Domagala, J., Roman, M., Górecka, A., Eds.; Productivity Press: New York, NY, USA, 2023; pp. 73–97. ISBN 9781032302966.
- Gayialis, S.P.; Kechagias, E.P.; Konstantakopoulos, G.D.; Papadopoulos, G.A. A Predictive Maintenance System for Reverse Supply Chain Operations. Logistics 2022, 6, 4.
- Goodarzian, F.; Kumar, V.; Ghasemi, P. A Set of Efficient Heuristics and Meta-Heuristics to Solve a Multi-Objective Pharmaceutical Supply Chain Network. Comput. Ind. Eng. 2021, 158, 107389.
- Liu, S.; Zhang, J.; Niu, B.; Liu, L.; He, X. A novel hybrid multi-criteria group decision-making approach with intuitionistic fuzzy sets to design reverse supply chains for COVID-19 medical waste recycling channels. Comput. Ind. Eng. 2022, 169, 108228.
- Sim, S.; Lai, P.; Tan, K.; Lee, H.; Sulaiman, C. Development and Validation of the Return and Disposal of Unused Medications Questionnaire (ReDiUM) in Malaysia. Asia Pac. J. Public Health 2018, 30, 727–749.
- EPIPEN Supply Information. Available online: https://www.epipen.com/about-epipen-and-generic/supply-information (accessed on 14 October 2022).
- Alhomoud, F. “Do not Let Medicines Go to Waste”—A Survey-Based Cross-Sectional Study of Pharmacists’ Waste-Reducing Activities Across Gulf Cooperation Council Countries. Front Pharmacol. 2020, 11, 1334.
- Makki, M.; Hassali, M.; Awaisu, A.; Hashmi, F. The Prevalence of Unused Medications in Homes. Pharmacy 2019, 7, 61.
- Inhaler Recycling. Available online: https://www.kentcht.nhs.uk/service/specialist-community-respiratory-service-east-kent/inhaler-recycling/ (accessed on 14 October 2022).
- Wheeler, A.; Spinks, J.; Kelly, F.; Bettington, E. Returning unwanted medicines to pharmacies: Prescribing to reduce waste. Aust. Prescr. 2018, 41, 78–81.
- Wu, P.; Juurlink, D. Unused prescription drugs should not be treated like leftovers. CMAJ 2014, 186, 815–816.
- Hsieh, D.; Lindrud, M.; Lu, X.; Zordan, C.; Tang, L.; Davies, M. A Process for Active Pharmaceutical Ingredient Recovery from Tablets Using Green Engineering Technology. Org. Process Res. Dev. 2017, 21, 1272–1285.
- Pratama, D.; Hsieh, W.; Elmaamoun, A.; Lee, H.; Lee, T. Recovery of Active Pharmaceutical Ingredients from Unused Solid Dosage-Form Drugs. ACS Omega 2020, 5, 29147–29157.
- De Filippis, P.; De Caprariis, B.; Scarsella, M.; Verdone, N. Energy recovery from unused and expired medicines. In Waste Management and the Environment VI; Popov, V., Itoh, H., Brebbia, C.A., Eds.; WIT Press: Southampton, UK, 2012; pp. 125–133. ISBN 978-1-84564-606-6.
- Hui, T.; Donyai, P.; McCrindle, R.; Sherratt, R. Enabling Medicine Reuse Using a Digital Time Temperature Humidity Sensor in an Internet of Pharmaceutical Things Concept. Sensors 2020, 20, 3080.
- Kongar, E.; Haznedaroglu, E.; Abdelghany, O.; Bahtiyar, M. A novel IT infrastructure for reverse logistics operations of end-of-life pharmaceutical products. Inf. Technol. Manag. 2015, 16, 51–65.
- El Mahboubi, F.; Bafleur, M.; Boitier, V.; Dilhac, J. Energy-Harvesting Powered Variable Storage Topology for Battery-Free Wireless Sensors. Technologies 2018, 6, 106.
- Herrojo, C.; Moras, M.; Paredes, F.; Núñez, A.; Ramon, E.; Mata-Contreras, J.; Martín, F. Very Low-Cost 80-Bit Chipless-RFID Tags Inkjet Printed on Ordinary Paper. Technologies 2018, 6, 52.
- Eldebiky, A.; Elsobky, M.; Richter, H.; Burghartz, J. Humidity and temperature sensor system demonstrator with NFC tag for HySiF applications. Adv. Radio Sci. 2018, 16, 109–116.
- Won, Y.; Kim, Y.; Lim, Y.; Moon, Y.; Lim, S. Development of Livestock Traceability System Based on Implantable RFID Sensor Tag with MFAN. J. Korea Inf. Commun. Soc. 2012, 37, 1318–1327.
- RFID Case Study: Descathlon Uses Taegos RFID Labels to Identify Millions of Items Worldwide. Available online: https://www.cisper.nl/en/case-studies/rfid-case-study-decathlon-uses-tageos-rfid-labels-to-identify-millions-of-items-worldwide (accessed on 17 January 2023).
- Pigini, D.; Conti, M. NFC-Based Traceability in the Food Chain. Sustainability 2017, 10, 1910.
