Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
The extracellular matrix (ECM) is a ubiquitous member of the body and is key to the maintenance of tissue and organ integrity. Initially thought to be a bystander in many cellular processes, the extracellular matrix has been shown to have diverse components that regulate and activate many cellular processes and ultimately influence cell phenotype.
- extracellular matrix
- tissues
- organs
- tumor progression
- collagens
- fibronectin
- integrins
- metastasis
- matrix metalloproteases
- cell adhesion
1. Introduction
Tissues and organs in the human body are composed of cells, biomolecules as well as the extracellular matrix [1]. The extracellular matrix (ECM) is key in many developmental stages from embryogenesis to adult development, and tissue repair as well as the maintenance of tissue and organ homeostasis [1,2]. Once synthesized in the cytoplasm, ECM components are secreted into the extracellular space where they are then modified further into final molecules [1,2]. The main recognized function of the ECM is the provision of physical support for cells within tissues and organs as well as availing the transportation of biomolecules such as growth factors and cytokines to cells. Recent reports indicate that the ECM is involved in the activation of several mechanosensitive signaling cascades and therefore impacts several cellular processes [3,4,5,6,7]. The two forms of the ECM are the interstitial ECM and the basement membrane.
The ECM is made up of several components that bond to form a complex network of different-sized molecules in a 3D unit (Figure 1). These ECM molecules are of different sizes, shapes, and spatial organization. Most tissues and organs have a specific type of ECM produced because of the differential expression of ECM genes as well as post-transcriptional splicing and post-translational modifications [8,9,10,11]. The ECM is in most cases in a state of flux, changing over time because of tissue development and disease [12,13,14]. Recent reports indicate that the ECM plays major role in disease progression and the development of chemoresistance [15,16,17,18]. Whilst cells synthesize the ECM, the ECM has been referred to as the ‘theatre’ within which cells interact with each other and biomolecules to effectively determine how cells behave [17,19]. Thus, cellular functions and phenotypes rely not just on gene expression but also on cues from the ECM.
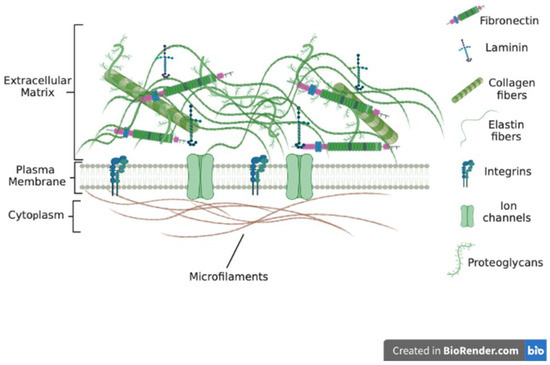
Figure 1. ECM molecules include collagens, laminins and fibronectin and proteoglycans. ECM is interconnected through various ECM molecules such as nidogen and perlecan. Proteoglycans bring collagen fibrils together to form large fibers.
ECM remodeling under normal physiological conditions is a tightly controlled complex process, with many proteins playing different roles to maintain homeostasis. The ECM also undergoes remodeling during tumorigenesis, with several reports indicating that it can promote tumorigenesis as well as be antitumorigenic [20,21,22]. In the early stages of tumor formation, stromal cells synthesize large amounts of ECM proteins in a bid to protect normal tissue from tumor cells [17,23,24]. This results in the stiffening of the tissue around the newly formed tumor. The stiffening of the ECM is due to enhanced collagen as well as hyaluronic acid deposition [25,26,27]. Chronic insult to a tissue results in the enhanced synthesis of ECM proteins, leading to a ‘fibrotic’ condition. Reports indicate that enhanced ECM deposition is positively correlated with tumor initiation and growth [28,29,30]. Circulating tumor cells have been shown to hone and colonize tissues and organs displaying increased ECM synthesis [31,32,33]. Both ECM proteins and the biomolecules found within the ECM have been identified as valuable markers for the diagnostic analysis of tumors [34,35]. This text discusses ECM composition, function, and remodeling processes and presents evidence of several ECM components suggested as novel therapeutic targets and currently being investigated or undergoing validation [36,37,38,39].
2. The Extracellular Matrix Macromolecules
The macromolecules found within tissues as well as organs that surround cells and provide tensile strength and other cues are what is termed the extracellular matrix. Various ‘omics’ studies have comprehensively identified ECM components, referred to as the ‘matrisome’, and more than 200 genes have been assigned in humans [40,41]. The macromolecules form a fibrillar network that interacts with cells and biomolecules to influence cell behavior in tissues and organs. The exact number of extracellular matrix macromolecules in the human body is unknown. Two major classes of the ECMs are known: the tissue-specific ECM and interstitial ECM. The type and composition of the ECM vary depending on several factors including the tissue and organ of the body. There are several classes of ECM macromolecules including fibrillar collagens, filament-forming collagens and glycoproteins. Other classes include elastic proteins as well as proteoglycans. Important classes include the collagens that constitute the connective tissue. Under normal physiological conditions, the ECM is highly organized into sheets that confer tensile strength to tissues and organs. However, ECM composition may differ under conditions such as stress and diseases. The ECM also provides cues to cells via tethered biomolecules and ligands to effectively influence cell behavior [42,43].
3. Collagens
The collagen family of proteins is the major component of the ECM and provides both mechanical strength and cues to cells and tissues. Reports indicate that collagens constitute around 90% of the ECM in humans [34,41]. Thus, collagens influence many cellular processes in the body including proliferation, migration, and adhesion [44]. Currently, about 28 proteins have been identified to belong to the collagen family [45]. Being the major proteins in the ECM, collagens undergo multiple changes and remodeling throughout an animal’s growth and development and in pathological conditions such as wound healing and cancers [46,47,48]. In addition, the synthesis of collagens requires modifications through the addition of disulfide bonds and other post-translational changes (Figure 2) [49,50]. Other ECM molecules also play a role in collagen synthesis and deposition. For example, the glycoprotein fibronectin is known to play a part in and influence the deposition and attachment of collagens in the extracellular space [51,52]. The overall structure and organization of the ECM are therefore results of the interaction between its constituents including collagens, glycoproteins, and other molecules [17,18,51,53,54]. Seven collagens have been grouped in the fibrillar class with type I collagen (or collagen type I) being a major component of this class. The other members include type II, type III, type V, type XI, type XXIV and type XXVII collagens [10,19,44,45]. Most collagens that form part of the basement membrane are grouped in the network-forming collagen class and these include type IV, type VIII, type X, type XV and type XVIII collagens [45]. Type VI and type XXVI collagens form the filament-forming class. The triple helical structure of some fibril-linked collagens can be interrupted and these include type IX, type XII, type XIV, type XVI, type XIX, and type XXII collagens [45]. Other collagens family members are found within or bound to membranes and these include type XIII, type XVII, type XXII, type XXIII and type XXV collagens [45].
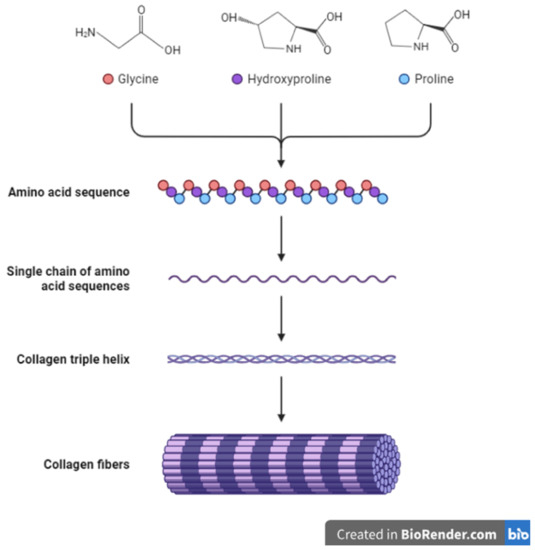
Figure 2. Schematic representation of collagen synthesis and structure.
Many studies have shown a link between changes in deposition and the amounts of collagens including the link between type I collagen and impaired development and the development of cancers [28,55]. Collagens found within the ECM in normal tissues can be highly uniform in orientation whilst in pathological conditions the orientation is varied [56,57]. Overall, the amounts of the different collagens in the ECM influence its properties from elasticity to availability of biomolecules such as growth factors and chemokines [58,59]. Collagens within the ECM also play other important roles within the body. For example, collagens are important within basement membranes where they contribute towards the separation of different layers of tissues. Increased collagen deposition within basement membranes can lead to membrane hardening disrupting the normal exchange of biomolecules and movement of cells [60,61]. In many pathological conditions such as cancer, basement membranes are thinner compared to normal tissues. This has been attributed to the reduced deposition of collagens including type IV, type XV, and type XIX collagens [62,63]. Indeed, several in vitro studies have also shown that collagen knockdown can enhance the migration of cancer cells [17,64,65].
4. Other Extracellular Matrix Macromolecules
A combination of proteins and carbohydrates make up glycoproteins and proteoglycans, with about 30 genes encoding these ECM components. The carbohydrates form repeating chains that are connected to a core made up of proteins. Proteoglycans are part of the glycoprotein family but are different from other glycoproteins in terms of their synthesis and structure. This ultimately influences their function in the body. Whilst glycoproteins have short and branched carbohydrate chains covalently linked to a protein core, the carbohydrate chains in proteoglycans are long and unbranched glycosaminoglycan chains also attached to a protein core [66,67]. Glycoproteins’ side chains create enough of a buffer to allow the ECM to resist stress and forces applied to the ECM [68,69]. In addition, glycoproteins are actively involved in regulating processes including proliferation and adhesion [69,70]. The glycosaminoglycan chains of proteoglycans are also negatively charged, allowing proteoglycans to impact the organization of other ECM constituents [70,71]. The negative charge of proteoglycans also allows the ECM as a whole to sequester growth factors and other biomolecules [72,73]. Due to their size and structure, proteoglycans can also participate in the binding of ligands to receptors, allowing cells to respond to various changes in extracellular cues. Several signaling pathways including the AKT-MEK and PI3-Akt cascades are activated through the participation of proteoglycans in bonding to various receptors [74,75]. The most well-known glycoproteins include fibronectin, fibrinogen, vitronectin, laminin, thrombospondins, periostin, and osteopontin. Among the well-known proteoglycans are decorin, aggrecan, and perlecan.
4.1. Laminin
A glycoprotein consisting of α, β, and γ chains that come together to form trimeric proteins, laminin or laminins is/are found within the basal lamina and contribute towards cell-specific functions including differentiation, adhesion, and migration [76,77]. Laminins as ECM glycoproteins play major roles in creating a link between the ECM and cells via binding to cellular receptors such as integrins. Thus, laminins are key to cellular migration and cancer cell invasive behavior. Currently, twelve mammalian chains (α, β and γ) have been identified, and these can combine in different amounts to form about sixty known laminins [78,79]. The α chains (200 to 400 kDa) are bigger than the β and γ chains (120 to 200 kDa) with the trimer being formed ranging from 400 to 800 kDa in size. Referred to as the ‘god molecule’ in some reports, trimeric laminin has a ‘cross’ shape formed as a result of its α-helical coiled-coil structure [80,81]. Laminins also bind to other ECM components including collagen type IV. In this case, laminins act as an intermediary or ‘glue’ between various ECM molecules within the basement membrane. Laminin polymerization is thought to be the main initiator of basement membrane assembly, placing laminin polymerization at the ‘center’ of cell function and tissue structure.
A well-known laminin molecule is laminin-332 (LN-332), which is formed by β3, α3, and γ2 chains, plays key roles in cellular migration and adhesion and contributes to tumor cell metastasis [76,77,82]. Laminin molecules are also implicated in maintaining stem cell self-renewal capabilities. For example, laminin-332 maintains CSCs’ self-renewal abilities and contributes to drug resistance [83]. Several reports show that the presence of laminins is closely linked to significantly lower patient survival in cancers such as colorectal and pancreatic cancer [82,84]. Laminins bind to other ECM proteins, and this promotes cell migration and adhesion as well as enhancing drug resistance [85,86]. For example, the binding of laminin-332 to the integrin α3β1 receptor increases resistance to gefitinib in hepatocellular carcinoma [87]. Various signaling cascades are also known to be activated through laminin–integrin interactions. For example, laminins’ interactions with integrins the cause activation of the mTOR cell survival signaling pathway [83,88].
4.2. Fibronectin
Structurally, fibronectin (FN) has several domains and is involved in the interactions between the ECM and cells. Fibronectin forms a fibrillar network and is key to cell differentiation, adhesion, and migration [89]. Fibronectin exists as a dimer of two molecules joined together via cysteine disulfide bonds. The assembly of fibronectin in the ECM occurs when it binds to α5β1 integrins via the RGD motif. Furthermore, the binding of fibronectin to integrins causes the clustering of integrin molecules, leading to increased levels of fibronectin molecules on the cell surface. Fibronectin-focal adhesion interactions alter the conformation of fibronectin, resulting in binding sites for other ECM molecules to be revealed. Fibronectin is therefore able to bind to collagens, laminins, and other proteins, allowing cells to adhere to the ECM and migrate [89]. Whilst it is a single gene-encoded protein, it has several isoforms resulting in proteins that form ECM fibrillar structures. Fibronectin binds to cell surface receptors and other ECM proteins such as collagens causing alterations to cells’ actin filaments, and this allows cells to migrate. Various reports show that fibronectin is key in cellular processes such as wound healing as well as in tumor growth [90]. Importantly, the adhesion of tumor cells to ECM proteins including fibronectin enhances the tumorigenic capacity of cancer cells as well as drug resistance [91,92]. Various studies have also associated increased fibronectin expression with tumor progression in various cancers [93,94,95]. Furthermore, clinical data associated enhanced fibronectin expression in tumors versus normal tissues with lower patient survival [90,96,97]. FN-induced migration was shown to be mediated via αvβ6 and α9β1 integrins in various cancers [90,98]. The binding of cancer cells to ECM proteins including fibronectin can protect cells from drug-induced apoptosis compared to cells attached to plastic [91]. Fibronectin-mediated reduction in apoptosis occurs via the inducement of cyclooxygenase-2 (COX-2) as well as the activation of integrin α5β1 [99,100]. In addition, various signaling cascades are activated when fibronectin binds to other ECM proteins [101]. The binding of cells to fibronectin also protects cells against many drug-induced states [83,102].
4.3. Periostin
Periostin is an adhesion-linked protein expressed as an ECM protein and produced within the periosteum as well as the periodontal ligaments [103,104]. It is a cell adhesion and nonstructural protein that functions to maintain tissue homeostasis, especially that of the tooth and bone tissues. It is mostly involved in many processes during development including cardiac development and healing but is expressed in low amounts in adult tissues [76,103]. Periostin mediates most of its effects via interacting with surface receptors such as integrins. The enhanced expression of periostin is associated with various pathological conditions including inflammation and disease, and many types of cancer including colon, lung, and breast cancer, and head and neck carcinomas [103,104]. Periostin is involved in regulating ECM–cell interactions via attachment to other ECM molecules including collagens, tenascin C, and fibronectin [104]. Periostin can bind to various integrins such as αvβ3, αvβ5, and α6β4, and thus influence the activation of many signaling cascades [105]. Some of the signaling cascades are Notch 1 and B-catenin signaling, which are important in cell differentiation and tissue specification. Various reports show that periostin is aberrantly expressed in pathological conditions such as arthritis, cancers, and fibrosis [104,105]. In various cancers, periostin has been shown to induce signaling cascades including PI3K-Akt through attaching to αvβ3 and αvβ5 integrins [106]. The presence of periostin enhances cancer cell proliferation and the process of EMT cancers such as gastric cancer [106,107]. Cancer cells showing resistance to various drugs including cisplatin and 5-fluorouracil (5-FU) also show increased periostin expression [104]. Thus, this suggests that increased periostin levels are correlated with drug resistance, tumor relapse, and tumor angiogenesis [108]. Periostin activates Akt phosphorylation in cancers including epithelial and ovarian cancer and carcinoma and this results in resistance, especially to paclitaxel [109]. Recent data suggest that periostin can be used as a prognostic marker in various cancers including pancreatic, ovarian, and esophageal cancers [110,111].
4.4. Hyaluronic Acid
Discovered almost a century ago by Karl Meyer and John Palmer whilst working on vitreous bovine eyes, hyaluronic acid is a glycosaminoglycan made up of N-acetylglucosamine and glucuronic acid repeats and is a common component of the ECM [112,113]. Hyaluronic acid is a long high-molecular-weight polymer with many hydroxyl moieties, allowing it to mix well in water [114,115]. Indeed, one of the main functions of hyaluronic acid is to retain water in various tissues [116,117]. Due to its size and the ability to form coils in water, hyaluronic acid can control the movement of biomolecules and ions within the ECM, allowing small molecules to pass whilst blocking the free movement or transport of larger biomolecules and substances [118]. Hyaluronic acid displays various unique properties such as biodegradability and great viscoelasticity, and has been utilized in various applications such as hydrogel formation and drug delivery systems [119,120]. Various studies have shown that hyaluronic acid plays crucial roles in cell migration and invasion through its interaction with receptors including CD44 and hyaluronan binding protein 4 [121,122].
This entry is adapted from the peer-reviewed paper 10.3390/biomimetics8020146
This entry is offline, you can click here to edit this entry!
